Control of action potential timing by intrinsic subthreshold oscillations in olfactory bulb output neurons
- PMID: 10594056
- PMCID: PMC6784925
- DOI: 10.1523/JNEUROSCI.19-24-10727.1999
Control of action potential timing by intrinsic subthreshold oscillations in olfactory bulb output neurons
Abstract
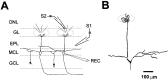
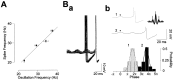
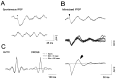
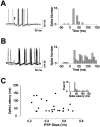
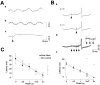
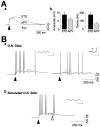
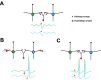
Rhythmic patterns of neuronal activity have been found at multiple levels of various sensory systems. In the olfactory bulb or the antennal lobe, oscillatory activity exhibits a broad range of frequencies and has been proposed to encode sensory information. However, the neural mechanisms underlying these oscillations are unknown. Bulbar oscillations might be an emergent network property arising from neuronal interactions and/or resulting from intrinsic oscillations in individual neurons. Here we show that mitral cells (output neurons of the olfactory bulb) display subthreshold oscillations of their membrane potential. These oscillations are mediated by tetrodotoxin-sensitive sodium currents and range in frequency from 10 to 50 Hz as a function of resting membrane potential. Because the voltage dependency of oscillation frequency was found to be similar to that for action potential generation, we studied how subthreshold oscillations could influence the timing of action potentials elicited by synaptic inputs. Indeed, we found that subthreshold oscillatory activity can trigger the precise occurrence of action potentials generated in response to EPSPs. Furthermore, IPSPs were found to set the phase of subthreshold oscillations and can lead to "rebound" spikes with a constant latency. Because intrinsic oscillations of membrane potential enable very precise temporal control of neuronal firing, we propose that these oscillations provide an effective means to synchronize mitral cell subpopulations during the processing of olfactory information.
Figures



References
-
- Alonso A, Llinás RR. Subthreshold Na+-dependent theta-like rhythmicity in stellate cells of entorhinal cortex layer II. Nature. 1989;342:175–177. - PubMed
-
- Amitai Y. Membrane potential oscillations underlying firing patterns in neocortical neurons. Neuroscience. 1994;63:151–161. - PubMed
-
- Bhalla US, Bower JM. Multiday recordings from olfactory bulb neurons in awake freely moving rats: spatially and temporally organized variability in odorant response properties. J Comput Neurosci. 1997;4:221–256. - PubMed
-
- Buzsaki G, Chrobak JJ. Temporal structure in spatially organized neuronal ensembles: a role for interneuronal networks. Curr Opin Neurobiol. 1995;5:504–510. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
