Sustained plateau activity precedes and can generate ictal-like discharges in low-Cl(-) medium in slices from rat piriform cortex
- PMID: 10594057
- PMCID: PMC6784944
- DOI: 10.1523/JNEUROSCI.19-24-10738.1999
Sustained plateau activity precedes and can generate ictal-like discharges in low-Cl(-) medium in slices from rat piriform cortex
Abstract
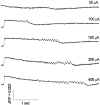
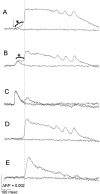
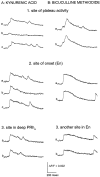
Interictal and ictal discharges represent two different forms of abnormal brain activity associated with epilepsy. Ictal discharges closely parallel seizure activity, but depending on the form of epilepsy, interictal discharges may or may not be correlated with the frequency, severity, and location of seizures. Recent voltage-imaging studies in slices of piriform cortex indicated that interictal-like discharges are generated in a two-stage process. The first stage consists of a sustained, low-amplitude depolarization (plateau activity) lasting the entire latent period prior to discharge onset. Plateau activity takes place at a site distinct from the site of discharge onset and serves to sustain and amplify activity initiated by an electrical stimulus. In the second stage a rapidly accelerating depolarization begins at the onset site and then spreads over a wide region. Here, we asked whether ictal-like discharges can be generated in a similar two-stage process. As with interictal-like activity, the first sign of an impending ictal-like discharge is a sustained depolarization with a plateau-like time course. The rapidly accelerating depolarization that signals the start of the actual discharge develops later at a separate onset site. As found previously with interictal-like discharges, local application of kynurenic acid to the plateau site blocked ictal-like discharges throughout the entire slice. However, in marked contrast to interictal-like activity, blockade of synaptic transmission at the onset site failed to block the ictal-like discharge. This indicates that interictal- and ictal-like discharges share a common pathway in the earliest stage of their generation and that their mechanisms subsequently diverge.
Figures




Similar articles
-
Sustained and accelerating activity at two discrete sites generate epileptiform discharges in slices of piriform cortex.J Neurosci. 1999 Feb 15;19(4):1294-306. doi: 10.1523/JNEUROSCI.19-04-01294.1999. J Neurosci. 1999. PMID: 9952407 Free PMC article.
-
Imaging epileptiform discharges in slices of piriform cortex with voltage-sensitive fluorescent dyes.Ann N Y Acad Sci. 2000 Jun;911:404-17. doi: 10.1111/j.1749-6632.2000.tb06740.x. Ann N Y Acad Sci. 2000. PMID: 10911888
-
Characteristics of plateau activity during the latent period prior to epileptiform discharges in slices from rat piriform cortex.J Neurophysiol. 2000 Feb;83(2):1088-98. doi: 10.1152/jn.2000.83.2.1088. J Neurophysiol. 2000. PMID: 10669520
-
Do interictal discharges promote or control seizures? Experimental evidence from an in vitro model of epileptiform discharge.Epilepsia. 2001;42 Suppl 3:2-4. doi: 10.1046/j.1528-1157.2001.042suppl.3002.x. Epilepsia. 2001. PMID: 11520313 Review.
-
Interictal-ictal interactions and limbic seizure generation.Rev Neurol (Paris). 1999 Jul;155(6-7):468-71. Rev Neurol (Paris). 1999. PMID: 10472661 Review.
Cited by
-
On the ictogenic properties of the piriform cortex in vitro.Epilepsia. 2012 Mar;53(3):459-68. doi: 10.1111/j.1528-1167.2012.03408.x. Epilepsia. 2012. PMID: 22372627 Free PMC article.
References
-
- Ayala GF, Matsumoto H, Gumnit RJ. Excitability changes and inhibitory mechanisms in neocortical neurons during seizures. J Neurophysiol. 1970;33:73–85. - PubMed
-
- Chamberlin NL, Dingledine R. GABAergic inhibition and the induction of spontaneous epileptiform activity by low chloride and high potassium in the hippocampal slice. Brain Res. 1988;445:12–18. - PubMed
-
- Demir R, Haberly LB, Jackson MB. Voltage imaging of epileptiform activity in slices from rat piriform cortex: onset and propagation. J Neurophysiol. 1998;80:2727–2742. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
