Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange
- PMID: 10594060
- PMCID: PMC6784962
- DOI: 10.1523/JNEUROSCI.19-24-10767.1999
Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange
Abstract
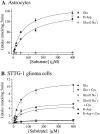
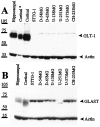
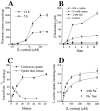
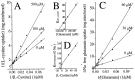
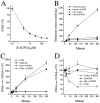
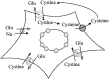
Elevated levels of extracellular glutamate ([Glu](o)) can induce seizures and cause excitotoxic neuronal cell death. This is normally prevented by astrocytic glutamate uptake. Neoplastic transformation of human astrocytes causes malignant gliomas, which are often associated with seizures and neuronal necrosis. Here, we show that Na(+)-dependent glutamate uptake in glioma cell lines derived from human tumors (STTG-1, D-54MG, D-65MG, U-373MG, U-251MG, U-138MG, and CH-235MG) is up to 100-fold lower than in astrocytes. Immunohistochemistry and subcellular fractionation show very low expression levels of the astrocytic glutamate transporter GLT-1 but normal expression levels of another glial glutamate transporter, GLAST. However, in glioma cells, essentially all GLAST protein was found in cell nuclei rather than the plasma membrane. Similarly, brain tissues from glioblastoma patients also display reduction of GLT-1 and mislocalization of GLAST. In glioma cell lines, over 50% of glutamate transport was Na(+)-independent and mediated by a cystine-glutamate exchanger (system x(c)(-)). Extracellular L-cystine dose-dependently induced glutamate release from glioma cells. Glutamate release was enhanced by extracellular glutamine and inhibited by (S)-4-carboxyphenylglycine, which blocked cystine-glutamate exchange. These data suggest that the unusual release of glutamate from glioma cells is caused by reduction-mislocalization of Na(+)-dependent glutamate transporters in conjunction with upregulation of cystine-glutamate exchange. The resulting glutamate release from glioma cells may contribute to tumor-associated necrosis and possibly to seizures in peritumoral brain tissue.
Figures


References
-
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. - PubMed
-
- Bannai S, Ishii T. Transport of cystine and cysteine and cell growth in cultured human diploid fibroblasts: effect of glutamate and homocysteate. J Cell Physiol. 1982;112:265–272. - PubMed
-
- Bannai S, Ishii T. A novel function of glutamine in cell culture: utilization of glutamine for the uptake of cystine in human fibroblasts. J Cell Physiol. 1988;137:360–366. - PubMed
-
- Bannai S, Kitamura E. Transport interaction of l-cystine and l-glutamate in human diploid fibroblasts in culture. J Biol Chem. 1980;255:2372–2376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
