The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar
- PMID: 10594061
- PMCID: PMC6784959
- DOI: 10.1523/JNEUROSCI.19-24-10778.1999
The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar
Abstract
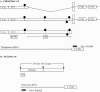
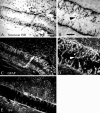
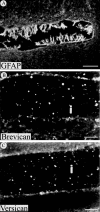
Chondroitin sulfate proteoglycans (CS-PGs) expressed by reactive astrocytes may contribute to the axon growth-inhibitory environment of the injured CNS. The specific potentially inhibitory CS-PGs present in areas of reactive gliosis, however, have yet to be thoroughly examined. In this study, we used immunohistochemistry, combined immunohistochemistry-in situ hybridization, immunoblot analysis, and reverse transcription-PCR to examine the expression of specific CS-PGs by reactive astrocytes in an in vivo model of reactive gliosis: that is, the glial scar, after cortical injury. Neurocan and phosphacan can be localized to reactive astrocytes 30 d after CNS injury, whereas brevican and versican are not expressed in the chronic glial scar. Neurocan is also expressed by astrocytes in primary cell culture. Relative to the amount present in cultured astrocytes or uninjured cortex, neurocan expression increases significantly in the glial scar resulting from cortical injury, including the re-expression of the neonatal isoform of neurocan. In contrast, phosphacan protein levels are decreased in the glial scar compared with the uninjured brain. Because these CS-PGs are capable of inhibiting neurite outgrowth in vitro, our data suggest that phosphacan and neurocan in areas of reactive gliosis may contribute to axonal regenerative failure after CNS injury.
Figures



References
-
- Ascher RA, Fidler PS, Rogers JH, Fawcett JW. TGF-β stimulates neurocan synthesis in cultured rat astrocytes. Soc Neurosci Abstr. 1998;24:56.
-
- Bovolenta P, Wandosell F, Nieto-Sampedro M. Characterization of a neurite outgrowth inhibitor expressed after CNS injury. Eur J Neurosci. 1993;5:454–465. - PubMed
-
- Bovolenta P, Fernaud-Espinosa I, Méndez-Otero R, Nieto-Sampedro M. Neurite outgrowth inhibitor of gliotic brain tissue. Mode of action and cellular localization, studied with specific monoclonal antibodies. Eur J Neurosci. 1997;9:977–989. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
