Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain
- PMID: 10594070
- PMCID: PMC6784931
- DOI: 10.1523/JNEUROSCI.19-24-10886.1999
Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain
Abstract
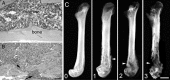
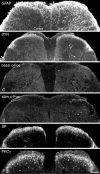
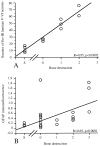
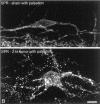
The cancer-related event that is most disruptive to the cancer patient's quality of life is pain. To begin to define the mechanisms that give rise to cancer pain, we examined the neurochemical changes that occur in the spinal cord and associated dorsal root ganglia in a murine model of bone cancer. Twenty-one days after intramedullary injection of osteolytic sarcoma cells into the femur, there was extensive bone destruction and invasion of the tumor into the periosteum, similar to that found in patients with osteolytic bone cancer. In the spinal cord, ipsilateral to the cancerous bone, there was a massive astrocyte hypertrophy without neuronal loss, an expression of dynorphin and c-Fos protein in neurons in the deep laminae of the dorsal horn. Additionally, normally non-noxious palpation of the bone with cancer induced behaviors indicative of pain, the internalization of the substance P receptor, and c-Fos expression in lamina I neurons. The alterations in the neurochemistry of the spinal cord and the sensitization of primary afferents were positively correlated with the extent of bone destruction and the growth of the tumor. This "neurochemical signature" of bone cancer pain appears unique when compared to changes that occur in persistent inflammatory or neuropathic pain states. Understanding the mechanisms by which the cancer cells induce this neurochemical reorganization may provide insight into peripheral factors that drive spinal cord plasticity and in the development of more effective treatments for cancer pain.
Figures


References
-
- Abbadie C, Besson J-M. c-Fos expression in rat lumbar spinal cord during the development of adjuvant-induced arthritis. Neuroscience. 1992;48:985–993. - PubMed
-
- Abbadie C, Besson J-M. c-Fos expression in rat lumbar spinal cord following peripheral stimulation in adjuvant-induced arthritis and in normal rats. Brain Res. 1993;607:195–204. - PubMed
-
- Abbadie C, Taylor BK, Peterson MA, Basbaum AI. Differential contribution of the two phases of the formalin test to the pattern of c-Fos expression in the rat spinal cord: studies with remifentanil and lidocaine. Pain. 1997a;69:101–110. - PubMed
-
- Adami S. Bisphosphonates in prostate carcinoma. Cancer. 1997;80:1674–1679. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
