Cerebral microvascular obstruction by fibrin is associated with upregulation of PAI-1 acutely after onset of focal embolic ischemia in rats
- PMID: 10594071
- PMCID: PMC6784928
- DOI: 10.1523/JNEUROSCI.19-24-10898.1999
Cerebral microvascular obstruction by fibrin is associated with upregulation of PAI-1 acutely after onset of focal embolic ischemia in rats
Abstract
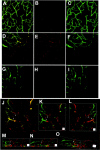
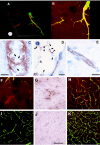
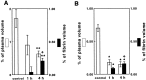
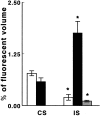
The mechanisms underlying cerebral microvascular perfusion deficit resulting from occlusion of the middle cerebral artery (MCA) require elucidation. We, therefore, tested the hypothesis that intravascular fibrin deposition in situ directly obstructs cerebral microcirculation and that local changes in type 1 plasminogen activator inhibitor (PAI-1) gene expression contribute to intravascular fibrin deposition after embolic MCA occlusion. Using laser-scanning confocal microscopy (LSCM) in combination with immunofluorescent staining, we simultaneously measured in three dimensions the distribution of microvascular plasma perfusion deficit and fibrin(ogen) immunoreactivity in a rat model of focal cerebral embolic ischemia (n = 12). In addition, using in situ hybridization and immunostaining, we analyzed expression of PAI-1 in ischemic brain (n = 13). A significant (p < 0.05) reduction of cerebral microvascular plasma perfusion accompanied a significant (p < 0.05) increase of intravascular and extravascular fibrin deposition in the ischemic lesion. Microvascular plasma perfusion deficit and fibrin deposition expanded concomitantly from the subcortex to the cortex during 1 and 4 hr of embolic MCA occlusion. Three-dimensional analysis revealed that intravascular fibrin deposition directly blocks microvascular plasma perfusion. Vascular plugs contained erythrocytes, polymorphonuclear leukocytes, and platelets enmeshed in fibrin. In situ hybridization demonstrated induction of PAI-1 mRNA in vascular endothelial cells in the ischemic region at 1 hr of ischemia. PAI-1 mRNA significantly increased at 4 hr of ischemia. Immunohistochemical staining showed the same pattern of increased PAI-1 antigen in the endothelial cells. These data demonstrate, for the first time, that progressive intravascular fibrin deposition directly blocks cerebral microvascular plasma perfusion in the ischemic region during acute focal cerebral embolic ischemia, and upregulation of the PAI-1 gene in the ischemic lesion may foster fibrin deposition through suppression of fibrinolysis.
Figures

References
-
- Adams H. Preliminary safety report of an ongoing dose-escalation trial Abciximab in acute ischemic stroke. Stroke. 1999;30:244.
-
- Bereczki D, Wei L, Otsuka T, Acuff V, Pettigrew K, Patlak C, Fenstermacher J. Hypoxia increases velocity of blood flow through parenchymal microvascular systems in rat brain. J Cereb Blood Flow Metab. 1992;13:475–486. - PubMed
-
- Braaten JV, Handt S, Jerome WG, Kirkpatrick J, Lewis JC, Hantgan RR. Regulation of fibrinolysis by platelet-released plasminogen activator inhibitor 1: light scattering and ultrastructural examination of lysis of a model platelet-fibrin thrombus. Blood. 1993;81:1290–1299. - PubMed
-
- Buchweitz-Milton E, Weiss HR. Perfused microvascular morphometry during middle cerebral artery occlusion. Am J Physiol. 1988;255:H623–H628. - PubMed
-
- Collen D, Lijnen HR. Basic and clinical aspects of fibrinolysis and thrombolysis. Blood. 1991;78:3114–3124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
