An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia
- PMID: 10594073
- PMCID: PMC6784955
- DOI: 10.1523/JNEUROSCI.19-24-10923.1999
An essential role of interleukin-1beta in mediating NF-kappaB activity and COX-2 transcription in cells of the blood-brain barrier in response to a systemic and localized inflammation but not during endotoxemia
Abstract
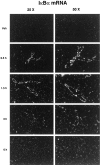
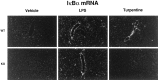
When released into the bloodstream, proinflammatory cytokines have the ability to trigger the transcription of different genes in cells of the blood-brain barrier (BBB), including members of the nuclear factor kappa B (NF-kappaB) family and cyclooxygenase-2 (COX-2), the limiting enzyme for the formation of prostaglandins (PGs). The present study investigated the possibility that interleukin-1beta (IL-1beta) plays an essential role in these events during a systemic inflammatory response. Both wild-type and IL-1beta-deficient mice were killed at different times after two different immunogenic stimuli, i.e., intraperitoneal lipopolysaccharide (LPS) injection and intramuscular turpentine injection, used here as a model of systemic localized inflammatory insult. The inhibitory factor kappaBalpha (IkappaBalpha, index of NF-kappaB activity) and COX-2 transcripts were detected throughout the brain by means of in situ hybridization. Systemic LPS injection caused a strong and rapid expression of IkappaBalpha in endothelial cells lining the BBB of large and small blood vessels and thereafter within parenchymal microglia across the brain. This treatment also provoked a transient expression of COX-2 along cells of the vascular system, and the expression pattern and intensity of the signal for both transcripts were essentially the same in wild-type and IL-1beta-deficient animals. In contrast, the induction of these genes that was quite selective to the cells of the BBB in response to intramuscularly turpentine insult was completely abolished in IL-1beta-deficient mice. Indeed, a late and prolonged expression of IkappaBalpha and COX-2 mRNAs was found along the cerebral blood vessels in response to the sterile and localized inflammation in wild-type mice, whereas such induction was absent in the brain of IL-1beta-deficient animals. These results indicate that IL-1beta has an obligatory role in the activation of NF-kappaB molecules and PGs within endothelial cells of the BBB in an experimental model of intramuscularly turpentine-induced inflammation but not during endotoxemia.
Figures


References
-
- Andersson J, Nagy S, Björk L, Abrams J, Holm S, Andersson U. Bacterial toxin-induced cytokine production studied at the single-cell level. Immunol Rev. 1992;127:69–96. - PubMed
-
- Arditi M, Zhou J, Torres M, Durden DL, Stins M, Kim KS. Lipopolysaccharide stimulates the tyrosine phosphorylation of mitogen-activated protein kinases p44, p42, and p41 in vascular endothelial cells in a soluble CD14-dependent manner. Role of protein tyrosine phosphorylation in lipopolysaccharide-induced stimulation of endothelial cells. J Immunol. 1995;155:3994–4003. - PubMed
-
- Baeuerle PA. Pro-inflammatory signaling: last pieces in the NF-kappa B puzzle. Curr Biol. 1998;8:R19–R22. - PubMed
-
- Baeuerle PA, Baltimore D. NF kappa B: ten years after. Cell. 1996;87:13–20. - PubMed
-
- Cao C, Matsumura K, Yamagata K, Watanabe Y. Endothelial cells of the rat brain vasculature express cyclooxygenase-2 mRNA in response to systemic interleukin-1 beta: a possible site of prostaglandin synthesis responsible for fever. Brain Res. 1996;733:263–272. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
