Wound-induced expression of the FAD7 gene is mediated by different regulatory domains of its promoter in leaves/stems and roots
- PMID: 10594110
- PMCID: PMC59490
- DOI: 10.1104/pp.121.4.1239
Wound-induced expression of the FAD7 gene is mediated by different regulatory domains of its promoter in leaves/stems and roots
Abstract
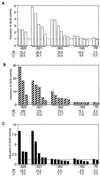
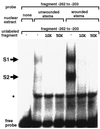
The FAD7 gene is expressed preferentially in the chlorophyllous tissues of unwounded plants. Wounding activates the expression of the FAD7 gene not only in chlorophyllous tissues, but also in nonchlorophyllous tissues of stems and roots. Our previous study suggested that wound-responsive transcriptional activation by the FAD7 promoter in leaves/stems and roots is brought about by a jasmonic acid (JA)-independent and JA-dependent signaling pathway, respectively. In this paper, we show that a specific region (from -259 to -198) in the FAD7 promoter is required for wound-activated expression of this gene in leaves and stems, while another region (from -521 to -363) is necessary not only for wound-activated but also for JA-responsive expression of this gene in roots. Thus, different regulatory regions of the FAD7 promoter mediate distinct wound-induced expression of this gene in leaves/stems and roots. Gel mobility shift assays revealed the wound-inducible DNA-binding activity to the -242/-223 region in both stem and leaf nuclear extracts. In fact, deletion of this region abolished wound response of the FAD7 promoter, suggesting the in vivo role of this site. Furthermore, we detected root nuclear factors interacting with the region from -433 to -363 of this promoter. Wounding and methyl jasmonate treatments induced differently these DNA-binding activities. These results suggest that different regulatory mechanisms mediate the wound-induced expression of the FAD7 gene in aerial and subterranean organs.
Figures




Similar articles
-
Wounding changes the spatial expression pattern of the arabidopsis plastid omega-3 fatty acid desaturase gene (FAD7) through different signal transduction pathways.Plant Cell. 1997 Oct;9(10):1701-12. doi: 10.1105/tpc.9.10.1701. Plant Cell. 1997. PMID: 9368411 Free PMC article.
-
Wound-response regulation of the sweet potato sporamin gene promoter region.Plant Mol Biol. 2002 Feb 1;48(3):223-31. doi: 10.1023/a:1013359227041. Plant Mol Biol. 2002. PMID: 11855724
-
Root-specific expression of a western white pine PR10 gene is mediated by different promoter regions in transgenic tobacco.Plant Mol Biol. 2003 May;52(1):103-20. doi: 10.1023/a:1023930326839. Plant Mol Biol. 2003. PMID: 12825693
-
Local and systemic signals in the wound response.Semin Cell Biol. 1993 Apr;4(2):103-11. doi: 10.1006/scel.1993.1013. Semin Cell Biol. 1993. PMID: 8318696 Review.
-
Multiple mechanisms behind plant bending.Nat Plants. 2023 Jan;9(1):13-21. doi: 10.1038/s41477-022-01310-y. Epub 2022 Dec 29. Nat Plants. 2023. PMID: 36581759 Review.
Cited by
-
Low temperature and light regulate delta 12 fatty acid desaturases (FAD2) at a transcriptional level in cotton (Gossypium hirsutum).J Exp Bot. 2008;59(8):2043-56. doi: 10.1093/jxb/ern065. Epub 2008 May 2. J Exp Bot. 2008. PMID: 18453533 Free PMC article.
-
Isolation and promoter analysis of a chalcone synthase gene PtrCHS4 from Populus trichocarpa.Plant Cell Rep. 2011 Sep;30(9):1661-71. doi: 10.1007/s00299-011-1075-1. Epub 2011 May 7. Plant Cell Rep. 2011. PMID: 21553109
-
Ntlim1, a PAL-box binding factor, controls promoter activity of the horseradish wound-inducible peroxidase gene.Plant Mol Biol. 2002 Aug;49(6):591-9. doi: 10.1023/a:1015504515492. Plant Mol Biol. 2002. PMID: 12081367
-
Dual positional specificity and expression of non-traditional lipoxygenase induced by wounding and methyl jasmonate in maize seedlings.Plant Mol Biol. 2003 Aug;52(6):1203-13. doi: 10.1023/b:plan.0000004331.94803.b0. Plant Mol Biol. 2003. PMID: 14682619
-
Different Cis-Regulatory Elements Control the Tissue-Specific Contribution of Plastid ω-3 Desaturases to Wounding and Hormone Responses.Front Plant Sci. 2021 Oct 27;12:727292. doi: 10.3389/fpls.2021.727292. eCollection 2021. Front Plant Sci. 2021. PMID: 34777414 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

