Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae
- PMID: 10594558
- PMCID: PMC1905437
- DOI: 10.1046/j.1365-2249.1999.01077.x
Contribution of serotype-specific IgG concentration, IgG subclasses and relative antibody avidity to opsonophagocytic activity against Streptococcus pneumoniae
Abstract
The contribution of serotype-specific IgG concentration, subclasses, and avidity to opsonophagocytic activity (OPA) against Streptococcus pneumoniae (Pnc) was evaluated in sera of adults and infants immunized with different pneumococcal vaccines. Antibody concentrations and avidities were measured by enzyme immunoassay (EIA) and OPAs by killing assay of Pnc. The most important factor contributing positively to OPA was the specific IgG level. In infants, a tendency to negative correlation was found between the concentration needed for killing of bacteria and avidity, suggesting that less antibodies of high rather than low avidity were required for killing. No such correlation was seen in adults. However, in adults the avidity was high already before vaccination and the variation was narrow. Thus, avidity was probably not a limiting factor influencing OPA. The effect of IgG2/IgG1 ratio on OPA was mostly negative but insignificant.
Figures

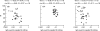
References
-
- Steinhoff MC, Edwards K, Keyserling H, Thoms ML, Johnson C, Madore D, Hogerman D. A randomized comparison of three bivalent Streptococcus pneumoniae glycoprotein conjugate vaccines in young children: effect of polysaccharide size and linkage characteristics. Pediatr Infect Dis J. 1994;13:368–72. - PubMed
-
- Käyhty H, Åhman H, Rönnberg P-R, Tillikainen R, Eskola J. Pneumococcal polysaccharide–meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J Infect Dis. 1995;172:1273–8. - PubMed
-
- Leach A, Ceesay SJ, Banya WA, Greenwood BM. Pilot trial of pentavalent pneumococcal polysaccharide/protein conjugate vaccine in Gambian infants. Pediatr Infect Dis J. 1996;15:333–9. - PubMed
-
- Dagan R, Melamed R, Zamir O, Leroy O. Safety and immunogenicity of tetravalent pneumococcal vaccines containing 6B, 14, 19F and 23F polysaccharides conjugated to either tetanus or diphtheria toxoid in young infants and their boosterability by native polysaccharide antigens. Pediatr Infect Dis J. 1997;16:1053–9. - PubMed
-
- Åhman H, Käyhty H, Lehtonen H, Leroy O, Froeschle J, Eskola J. Streptococcus pneumoniae capsular polysaccharide–diphtheria toxoid conjugate vaccine is immunogenic in early infancy and able to induce immunologic memory. Pediatr Infect Dis J. 1998;17:211–6. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

