Adhesion to fibronectin promotes the activation of the p125(FAK)/Zap-70complex in human T cells
- PMID: 10594689
- PMCID: PMC2326958
- DOI: 10.1046/j.1365-2567.1999.00917.x
Adhesion to fibronectin promotes the activation of the p125(FAK)/Zap-70complex in human T cells
Abstract
The beta1 integrins are a family of heterodimeric adhesion receptors involved in cell-to-cell contacts and cell-to-extracellular matrix interactions. Through their adhesive role, integrins participate in transduction of outside/inside signals and contribute to trigger a multitude of cellular events such as differentiation, cell activation, and motility. The fibronectin integrin receptors, alpha4beta1 and alpha5beta1, can function as costimulatory molecules in T-cell receptor (TCR)-dependent T-cell activation. In the current study the Jurkat T-cell line was used as a model system to investigate the TCR-independent role of cell adhesion to fibronectin in the activation of Zap-70, a central molecule in the signalling events in T cells. Upon adhesion to plastic immobilized fibronectin but not to bovine serum albumin (BSA) the phosphorylation of p125FAK, a protein kinase that localizes to focal adhesion sites, was induced. Moreover, clustering of fibronectin receptors led to the detection of a p125FAK/Zap-70 complex. Finally, while the complex between fak-B, another protein kinase localized to focal adhesion sites, and Zap-70 was detected in cells plated either on BSA or on fibronectin, the formation of the p125FAK/Zap-70 complex appeared specifically induced following fibronectin-mediated integrin clustering. These data suggest the existence of a high degree of specificity when the members of the beta1 integrin family mediate signalling pathways in T cells.
Figures
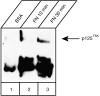

References
-
- Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science. 1995;268:233. - PubMed
-
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins – emerging paradigms of signal‐transduction. Annu Rev Cell Dev Biol. 1995;11:549. - PubMed
-
- Yamada KM, Geiger B. Molecular interactions in cell adhesion complexes. Curr Opin Cell Biol. 1997;9:76. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

