Some Bence-Jones proteins enter cultured renal tubular cells, reach nuclei and induce cell death
- PMID: 10594692
- PMCID: PMC2326963
- DOI: 10.1046/j.1365-2567.1999.00898.x
Some Bence-Jones proteins enter cultured renal tubular cells, reach nuclei and induce cell death
Abstract
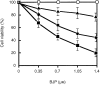
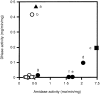
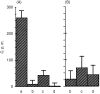
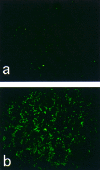
Eighteen monoclonal Bence-Jones proteins (BJPs) were examined for their effects on cultured LLC-PK1 (porcine kidney proximal tubule) cells as well as for their amidase and DNase activities. Five proteins were found to enter the cell and to gain access to the nucleus without degradation of epitopes. Intranuclear BJPs ultimately induced DNA fragmentation and cell death. BJPs with relatively high amidase activity were cytotoxic. On the other hand, three of four BJPs with DNase activity had a cytocidal effect on cultured cells; the remaining BJP, which had a relatively high DNase activity but a very low amidase activity, failed to enter the cell and was not cytotoxic in vitro. These results suggest that catalytic and cytotoxic activities of some BJPs may make a significant contribution, in a substantial proportion of myeloma patients, to the development and/or deterioration of the disease.
Figures


References
-
- Paul S. Natural catalytic antibodies. Mol Biotechnol. 1996;5:197. - PubMed
-
- Paul S. Antibody catalysis. In: Paul P, editor. Contemporary Immunology: Autoimmune Diseases. Totowa, NJ: Humana Press; 1998. p. 221.
-
- Paul S, Li L, Kalaga RA, et al. Natural catalytic antibodies: peptide‐hydrolyzing activities of Bence Jones proteins and VL fragment. J Biol Chem. 1995;270:15257. - PubMed
-
- Li L, Paul S, Tyutyulkova S, et al. Catalytic activity of anti‐thyroglobulin antibodies. J Immunol. 1995;154:3328. - PubMed
-
- Shuster AM, Gololobov GV, Kvashuk OA, et al. DNA hydrolyzing autoantibodies. Science. 1992;256:665. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

