Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis
- PMID: 10595922
- PMCID: PMC1866950
- DOI: 10.1016/S0002-9440(10)65511-3
Blood-brain barrier tight junction disruption in human immunodeficiency virus-1 encephalitis
Abstract
The blood-brain barrier (BBB) plays a critical role in regulating cell trafficking through the central nervous system (CNS) due to several unique anatomical features, including the presence of interendothelial tight junctions that form impermeable seals between the cells. Previous studies have demonstrated BBB perturbations during human immunodeficiency virus encephalitis (HIVE); however, the basis of these permeability changes and its relationship to infiltration of human immunodeficiency virus type 1 (HIV-1)-infected monocytes, a critical event in the pathogenesis of the disease, remains unclear. In this study, we examined CNS tissue from HIV-1-seronegative patients and HIV-1-infected patients, both with and without encephalitis, for alterations in BBB integrity via immunohistochemical analysis of the tight junction membrane proteins, occludin and zonula occludens-1 (ZO-1). Significant tight junction disruption (P < 0.001), as demonstrated by fragmentation or absence of immunoreactivity for occludin and ZO-1, was observed within vessels from subcortical white matter, basal ganglia, and, to a lesser extent, cortical gray matter in patients who died with HIVE. These alterations were also associated with accumulation of activated, HIV-1-infected brain macrophages, fibrinogen leakage, and marked astrocytosis. In contrast, no significant changes (P > 0.05) were observed in cerebellar tissue from patients with HIVE compared to HIV-seronegative patients or HIV-1-infected patients without encephalitis. Our findings demonstrate that tight junction disruption is a key feature of HIVE that occurs in regions of histopathological alterations in association with perivascular accumulation of activated HIV-1-infected macrophages, serum protein extravasation, and marked astrocytosis. We propose that disruption of this key BBB structure serves as the main route of HIV-1-infected monocyte entry into the CNS.
Figures

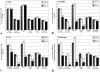
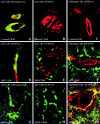
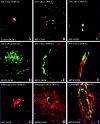
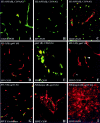
Similar articles
-
Blood-brain barrier disruption in simian immunodeficiency virus encephalitis.Neuropathol Appl Neurobiol. 2000 Oct;26(5):454-62. doi: 10.1046/j.1365-2990.2000.00275.x. Neuropathol Appl Neurobiol. 2000. PMID: 11054186
-
HIV-1 Tat protein alters tight junction protein expression and distribution in cultured brain endothelial cells.J Neurosci Res. 2003 Oct 15;74(2):255-65. doi: 10.1002/jnr.10762. J Neurosci Res. 2003. PMID: 14515355
-
Association of FAK activation with lentivirus-induced disruption of blood-brain barrier tight junction-associated ZO-1 protein organization.J Neurovirol. 2009 Jul;15(4):312-23. doi: 10.1080/13550280902998413. J Neurovirol. 2009. PMID: 19521898 Free PMC article.
-
The neuropathology of adult HIV infection.Rev Neurol (Paris). 1998 Dec;154(12):816-29. Rev Neurol (Paris). 1998. PMID: 9932303 Review.
-
HIV-1-infection in the CNS. A pathogenesis of some neurological syndromes in the light of recent investigations.Folia Neuropathol. 1998;36(4):211-6. Folia Neuropathol. 1998. PMID: 10079602 Review.
Cited by
-
HIV-1 Nef breaches placental barrier in rat model.PLoS One. 2012;7(12):e51518. doi: 10.1371/journal.pone.0051518. Epub 2012 Dec 11. PLoS One. 2012. PMID: 23240037 Free PMC article.
-
Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease.J Clin Invest. 2012 Jul;122(7):2454-68. doi: 10.1172/JCI60842. Epub 2012 Jun 1. J Clin Invest. 2012. PMID: 22653056 Free PMC article.
-
Signalling crosstalk in FGF2-mediated protection of endothelial cells from HIV-gp120.BMC Neurosci. 2005 Feb 2;6:8. doi: 10.1186/1471-2202-6-8. BMC Neurosci. 2005. PMID: 15689238 Free PMC article.
-
Proteomic and cellular localisation studies suggest non-tight junction cytoplasmic and nuclear roles for occludin in astrocytes.Eur J Neurosci. 2018 Jun;47(12):1444-1456. doi: 10.1111/ejn.13933. Epub 2018 May 30. Eur J Neurosci. 2018. PMID: 29738614 Free PMC article.
-
Brain vascular heterogeneity: implications for disease pathogenesis and design of in vitro blood-brain barrier models.Fluids Barriers CNS. 2018 Apr 23;15(1):12. doi: 10.1186/s12987-018-0097-2. Fluids Barriers CNS. 2018. PMID: 29688865 Free PMC article. Review.
References
-
- Janssen RS, Cornblath DR, Epstein LG, Foa RP, McArthur JC, Price RW, Asbury AKK, Beckett A, Benson DF, Bridge TP, Leventhal CM, Satz P, Saykin AJ, Sidtiss JJ, Tross S: Nomenclature and research case definitions for neurological manifestations of human immunodeficiency virus type-1 (HIV-1) infection. Neurology 1991, 41:778-785 - PubMed
-
- Navia BA, Jordan BD, Price RW: The AIDS dementia complex. I. Clinical features. Ann Neurol 1986, 19:517-524 - PubMed
-
- Price RW, Brew B, Sidtis J, Rosenblum M, Schneck AC, Cleary P: The brain and AIDS: central nervous system HIV-1 infection and AIDS dementia complex. Science 1988, 239:586-592 - PubMed
-
- : AAN Task Force: Nomenclature and research case definitions for neurologic manifestations of human immunodeficiency virus-type 1 (HIV-1) infection. Report of a Working Group of the American Academy of Neurology AIDS Task Force. Neurology 1991, 41:778-785 - PubMed
-
- Navia BA, Cho ES, Petito CK, Price PW: The AIDS dementia complex. II. Neuropathology. Ann Neurol 1986, 19:525-535 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

