Altered centrosome structure is associated with abnormal mitoses in human breast tumors
- PMID: 10595924
- PMCID: PMC1866918
- DOI: 10.1016/S0002-9440(10)65513-7
Altered centrosome structure is associated with abnormal mitoses in human breast tumors
Abstract
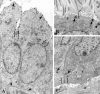
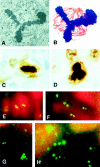
Centrosomes are the major microtubule organizing center in mammalian cells and establish the spindle poles during mitosis. Centrosome defects have been implicated in disease and tumor progression and have been associated with nullizygosity of the p53 tumor suppressor gene. In the present ultrastructural analysis of 31 human breast tumors, we found that centrosomes of most tumors had significant alterations compared to centrosomes of normal breast tissue. These alterations in included 1) supernumerary centrioles, 2) excess pericentriolar material, 3) disrupted centriole barrel structure, 4) unincorporated microtubule complexes, 5) centrioles of unusual length, 6) centrioles functioning as ciliary basal bodies, and 7) mispositioned centrosomes. These alterations are associated with changes in cell polarity, changes in cell and tissue differentiation, and chromosome missegregation through multipolar mitoses. Significantly, the presence of excess pericentriolar material was associated with the highest frequency of abnormal mitoses. Centrosome abnormalities may confer a mutator phenotype to tumors, occasionally yielding cells with a selective advantage that emerge and thrive, thus leading the tumor to a more aggressive state.
Figures





Similar articles
-
The contribution of epigenetic changes to abnormal centrosomes and genomic instability in breast cancer.J Mammary Gland Biol Neoplasia. 2001 Apr;6(2):203-12. doi: 10.1023/a:1011312808421. J Mammary Gland Biol Neoplasia. 2001. PMID: 11501580 Review.
-
Centrosome-centriole abnormalities are markers for abnormal cell divisions and cancer in the transgenic adenocarcinoma mouse prostate (TRAMP) model.Biol Cell. 2000 Aug;92(5):331-40. doi: 10.1016/s0248-4900(00)01079-0. Biol Cell. 2000. PMID: 11071042
-
Mitosis in the human embryo: the vital role of the sperm centrosome (centriole).Histol Histopathol. 1997 Jul;12(3):827-56. Histol Histopathol. 1997. PMID: 9225167 Review.
-
Centrosome hypertrophy in human breast tumors: implications for genomic stability and cell polarity.Proc Natl Acad Sci U S A. 1998 Mar 17;95(6):2950-5. doi: 10.1073/pnas.95.6.2950. Proc Natl Acad Sci U S A. 1998. PMID: 9501196 Free PMC article.
-
Centrosome amplification and the origin of chromosomal instability in breast cancer.J Mammary Gland Biol Neoplasia. 2004 Jul;9(3):275-83. doi: 10.1023/B:JOMG.0000048774.27697.30. J Mammary Gland Biol Neoplasia. 2004. PMID: 15557800 Review.
Cited by
-
Silencing DNA Polymerase β Induces Aneuploidy as a Biomarker of Poor Prognosis in Oral Squamous Cell Cancer.Int J Mol Sci. 2021 Feb 27;22(5):2402. doi: 10.3390/ijms22052402. Int J Mol Sci. 2021. PMID: 33673690 Free PMC article.
-
Krüppel-like factor 4 prevents centrosome amplification following gamma-irradiation-induced DNA damage.Oncogene. 2005 Jun 9;24(25):4017-25. doi: 10.1038/sj.onc.1208576. Oncogene. 2005. PMID: 15806166 Free PMC article.
-
The contribution of epigenetic changes to abnormal centrosomes and genomic instability in breast cancer.J Mammary Gland Biol Neoplasia. 2001 Apr;6(2):203-12. doi: 10.1023/a:1011312808421. J Mammary Gland Biol Neoplasia. 2001. PMID: 11501580 Review.
-
Centrosome amplification is a frequent event in circulating tumor cells from subjects with metastatic breast cancer.Mol Oncol. 2020 Aug;14(8):1898-1909. doi: 10.1002/1878-0261.12687. Epub 2020 May 19. Mol Oncol. 2020. PMID: 32255253 Free PMC article.
-
Structural centrosome aberrations favor proliferation by abrogating microtubule-dependent tissue integrity of breast epithelial mammospheres.Oncogene. 2016 May;35(21):2711-22. doi: 10.1038/onc.2015.332. Epub 2015 Sep 14. Oncogene. 2016. PMID: 26364601 Free PMC article.
References
-
- Hartwell LH, Weinert TA: Checkpoints: controls that ensure the order of cell cycle events. Science 1989, 246:629-634 - PubMed
-
- Boveri T: Zur Frage der Emtstehung Maligner Tumoren. Fischer Verlag, Jena, 1914. English translation by Boveri M, The Origin of Malignant Tumors. Baltimore, Waverly Press, 1929
-
- Levine DS, Sanchez CA, Rabinovitch PS, Reid BJ: Formation of the tetraploid intermediate is associated with the development of cells with more than four centrioles in the elastase-simian virus 40 tumor antigen transgenic mouse model of pancreatic cancer. Proc Natl Acad Sci USA 1991, 88:6427-6431 - PMC - PubMed
-
- Cross SM, Sanchez CA, Morgan CA, Schimke MK, Ramel S, Idzerda RL, Raskind WH, Reid BJ: A p53-dependent mouse spindle checkpoint. Science 1995, 267:1353-1356 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

