Human vascular adhesion protein-1 in smooth muscle cells
- PMID: 10595925
- PMCID: PMC1866916
- DOI: 10.1016/S0002-9440(10)65514-9
Human vascular adhesion protein-1 in smooth muscle cells
Abstract
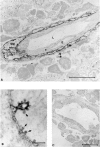
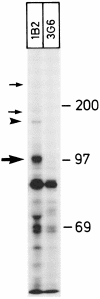
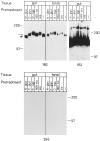
Human vascular adhesion protein-1 (VAP-1) is a dual-function molecule with adhesive and enzymatic properties. In addition to synthesis in endothelial cells, where it mediates lymphocyte binding, VAP-1 is expressed in smooth muscle cells. Here we studied the expression, biochemical structure, and function of VAP-1 in muscle cells and compared it to those in endothelial cells. VAP-1 is expressed on the plasma membrane of all types of smooth muscle cells, but it is completely absent from cardiac and skeletal muscle cells. In tumors, VAP-1 is retained on all leiomyoma cells, whereas it is lost in half of leiomyosarcoma samples. In smooth muscle VAP-1 predominantly exists as a approximately 165-kd homodimeric glycoprotein, but a trimeric (approximately 250 kd) form of VAP-1 is also found. It contains N-linked oligosaccharide side chains and abundant sialic acid decorations. In comparison, in endothelial cells dimeric VAP-1 is larger, no trimeric forms are found, and VAP-1 does not have N-glycanase-sensitive oligosaccharides. Unlike endothelial VAP-1, VAP-1 localized on smooth muscle cells does not support binding of lymphocytes. Instead, it deaminates exogenous and endogenous primary amines. In conclusion, VAP-1 in smooth muscle cells is structurally and functionally distinct from VAP-1 present on endothelial cells.
Figures






Similar articles
-
Different forms of human vascular adhesion protein-1 (VAP-1) in blood vessels in vivo and in cultured endothelial cells: implications for lymphocyte-endothelial cell adhesion models.Eur J Immunol. 1995 Oct;25(10):2803-12. doi: 10.1002/eji.1830251014. Eur J Immunol. 1995. PMID: 7589075
-
Lymphocyte binding to vascular endothelium in inflamed skin revisited: a central role for vascular adhesion protein-1 (VAP-1).Eur J Immunol. 1996 Apr;26(4):825-33. doi: 10.1002/eji.1830260415. Eur J Immunol. 1996. PMID: 8625974
-
Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule.J Exp Med. 1998 Jul 6;188(1):17-27. doi: 10.1084/jem.188.1.17. J Exp Med. 1998. PMID: 9653080 Free PMC article.
-
A novel endothelial cell molecule mediating lymphocyte binding in humans.Behring Inst Mitt. 1993 Aug;(92):36-43. Behring Inst Mitt. 1993. PMID: 8250815 Review.
-
[Vascular adhesion protein-1 (VAP-1) in inflammatory process].Przegl Lek. 2006;63(4):220-2. Przegl Lek. 2006. PMID: 17083159 Review. Polish.
Cited by
-
Arterial vascular cell line expressing SSAO: a new tool to study the pathophysiology of vascular amine oxidases.J Neural Transm (Vienna). 2013 Jun;120(6):1005-13. doi: 10.1007/s00702-013-1015-z. Epub 2013 Apr 2. J Neural Transm (Vienna). 2013. PMID: 23546801
-
Vectorial proteomics reveal targeting, phosphorylation and specific fragmentation of polymerase I and transcript release factor (PTRF) at the surface of caveolae in human adipocytes.Biochem J. 2004 Oct 15;383(Pt 2):237-48. doi: 10.1042/BJ20040647. Biochem J. 2004. PMID: 15242332 Free PMC article.
-
Time-dependent activation of the semicarbazide-sensitive amine oxidase (SSAO) from ox lung microsomes.Biochem J. 2000 Nov 1;351 Pt 3(Pt 3):789-94. Biochem J. 2000. PMID: 11042135 Free PMC article.
-
Multivalent Interactions of Human Primary Amine Oxidase with the V and C22 Domains of Sialic Acid-Binding Immunoglobulin-Like Lectin-9 Regulate Its Binding and Amine Oxidase Activity.PLoS One. 2016 Nov 28;11(11):e0166935. doi: 10.1371/journal.pone.0166935. eCollection 2016. PLoS One. 2016. PMID: 27893774 Free PMC article.
-
The role of VAP-1 in cardiovascular disease: a review.Front Cardiovasc Med. 2025 May 19;12:1549157. doi: 10.3389/fcvm.2025.1549157. eCollection 2025. Front Cardiovasc Med. 2025. PMID: 40458597 Free PMC article. Review.
References
-
- Salmi M, Jalkanen S: A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science 1992, 257:1407-1409 - PubMed
-
- Lyles GA: Mammalian plasma and tissue-bound semicarbazide-sensitive amine oxidases: biochemical, pharmacological and toxicological aspects. Int J Biochem Cell Biol 1996, 28:259-274 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

