Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis
- PMID: 10595928
- PMCID: PMC1866955
- DOI: 10.1016/S0002-9440(10)65517-4
Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis
Abstract
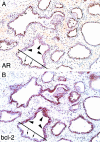
Proliferation in the setting of longstanding chronic inflammation appears to predispose to carcinoma in the liver, large bowel, urinary bladder, and gastric mucosa. Focal prostatic atrophy, which is associated with chronic inflammation, is highly proliferative (Ruska et al, Am J Surg Pathol 1998, 22:1073-1077); thus the focus of this study was to more fully characterize the phenotype of the atrophic cells to assess the feasibility of the proposal that they may be targets of neoplastic transformation. The pi-class glutathione S-transferase (GSTP1), a carcinogen-detoxifying enzyme, is not expressed in >90% of prostate carcinomas (CaPs). GSTP1 promoter hypermethylation, which appears to permanently silence transcription, is the most frequently detected genomic alteration in CaP (Lee et al, Proc Natl Acad Sci USA 1994, 91:11733-11737; >90% of cases). In high-grade prostatic intraepithelial neoplasia (PIN), this alteration is present in at least 70% of cases (Brooks et al, Cancer Epidemiol Biomarkers Prev, 1998, 7:531-536). Although normal-appearing prostate secretory cells rarely express GSTP1, they remain capable of expression, inasmuch as GSTP1 promoter hypermethylation is not detected in normal prostate. Fifty-five lesions from paraffin-embedded prostatectomy specimens (n = 42) were stained for GSTP1, using immunohistochemistry. Adjacent sections were stained for p27(Kip1), Ki-67, androgen receptor (AR), prostate-specific antigen (PSA), prostate-specific acid phosphatase (PSAP), Bcl-2, and basal cell-specific cytokeratins (34betaE12). With normal prostate epithelium as the internal standard, staining was scored for each marker in the atrophic epithelium. The lesions showed two cell types, basal cells staining positive for 34betaE12, and atrophic secretory-type cells staining weakly negative for 34betaE12. All lesions showed elevated levels of Bcl-2 in many of the secretory-type cells. All lesions had an elevated staining index for the proliferation marker Ki-67 in the secretory layer and decreased expression of p27(Kip1), a finding reminiscent of high-grade PIN (De Marzo et al, Am J Pathol 1998, 153:911-919). Consistent with partial secretory cell differentiation, the luminal cells showed weak to moderate staining for androgen receptor and the secretory proteins PSA and PSAP. All atrophic lesions showed elevated GSTP1 expression in many of the luminal secretory-type cells. Because all lesions are hyperproliferative, are associated with inflammation, and have the distinct morphological appearance recognized as prostatic atrophy, we suggest the term "proliferative inflammatory atrophy" (PIA). Elevated levels of GSTP1 may reflect its inducible nature in secretory cells, possibly in response to increased electrophile or oxidant stress. Elevated Bcl-2 expression may be responsible for the very low apoptotic rate in PIA and is consistent with the conclusion that PIA is a regenerative lesion. We discuss our proposal to integrate the atrophy and high-grade PIN hypotheses of prostate carcinogenesis by suggesting that atrophy may give rise to carcinoma either directly, as previously postulated, or indirectly by first developing into high-grade PIN.
Figures




Comment in
-
Inflammatory pathogenesis of prostate cancer and celecoxib.J Clin Oncol. 2010 Apr 20;28(12):e197; author reply e198. doi: 10.1200/JCO.2009.27.1098. Epub 2010 Feb 22. J Clin Oncol. 2010. PMID: 20177014 No abstract available.
References
-
- Ames BN: Mutagenesis and carcinogenesis: endogenous and exogenous factors. Environ Mol Mutagen 1989, 14:66-77 - PubMed
-
- Weitzman SA, Gordon LI: Inflammation and cancer: role of phagocyte-generated oxidants in carcinogenesis. Blood 1990, 76:655-663 - PubMed
-
- Bartsch H, Frank N: Blocking the endogenous formation of N-nitroso compounds and related carcinogens. IARC Sci Publ 1996, 139:189-201 - PubMed
-
- Smith CJ, Gardner WA, Jr: Inflammation-proliferation: possible relationships in the prostate. Prog Clin Biol Res 1987, 239:317-325 - PubMed
-
- Bennett BD, Richardson PH, Gardner WA: Histopathology and cytology of prostatitis. Lepor H Lawson RK eds. Prostate Diseases. 1993, :pp 399-414 W. B. Saunders Company, Philadelphia,
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

