Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast
- PMID: 10595936
- PMCID: PMC1866930
- DOI: 10.1016/S0002-9440(10)65525-3
Gastrin-releasing peptide receptors in non-neoplastic and neoplastic human breast
Abstract
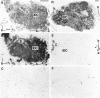
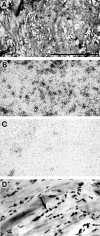
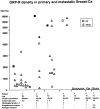
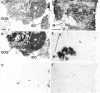
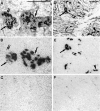
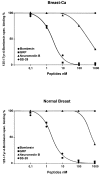
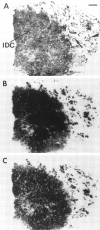
The regulatory peptide gastrin-releasing peptide (GRP) may play a role in human cancer as a stimulatory growth factor. To understand the potential role of GRP in human breast cancer, we have evaluated GRP receptor expression in human non-neoplastic and neoplastic breast tissues and in axillary lymph node metastases, using in vitro receptor autoradiography on tissue sections with [(125)I]Tyr(4)-bombesin and with [(125)I]D-Tyr(6), beta Ala(11), Phe(13), Nle(14)-bombesin(6-14) as radioligands. GRP receptors were detected, often in high density, in neoplastic epithelial mammary cells in 29 of 46 invasive ductal carcinomas, in 11 of 17 ductal carcinomas in situ, in 1 of 4 invasive lobular carcinomas, in 1 of 2 lobular carcinomas in situ, and in 1 mucinous and 1 tubular carcinoma. A heterogeneous GRP receptor distribution was found in the neoplastic tissue samples in 32 of 52 cases with invasive carcinoma and 12 of 19 cases with carcinoma in situ. The lymph node metastases (n = 33) from those primary carcinomas expressing GRP receptors were all positive, whereas surrounding lymphoreticular tissue was negative. GRP receptors were also present in high density but with heterogeneous distribution in ducts and lobules from all available breast tissue samples (n = 23). All of the receptors corresponded to the GRP receptor subtype of bombesin receptors, having high affinity for GRP and bombesin and lower affinity for neuromedin B. All tissues expressing GRP receptors were identified similarly with both radioligands. These data describe not only a high percentage of GRP receptor-positive neoplastic breast tissues but also for the first time a ubiquitous GRP receptor expression in nonneoplastic human breast tissue. Apart from suggesting a role of GRP in breast physiology, these data represent the molecular basis for potential clinical applications of GRP analogs such as GRP receptor scintigraphy, radiotherapy, or chemotherapy.
Figures

References
-
- Reubi JC: Neuropeptide receptors in health and disease: the molecular basis for in vivo imaging. J Nucl Med 1995, 36:1825-1835 - PubMed
-
- Reubi JC: In vitro identification of vasoactive intestinal peptide receptors in human tumors: implications for tumor imaging. J Nucl Med 1995, 36:1846-1853 - PubMed
-
- Reubi JC, Schaer JC, Waser B: Cholecystokinin(CCK)-A, and CCK-B/gastrin receptors in human tumors. Cancer Res 1997, 57:1377-1386 - PubMed
-
- Markwalder R, Reubi JC: Gastrin-releasing peptide receptors in the human prostate: relation to neoplastic transformation. Cancer Res 1999, 59:1152-1159 - PubMed
-
- Krenning EP, Kwekkeboom DJ, Pauwels S, Kvols LK, Reubi JC: Somatostatin Receptor Scintigraphy. Freeman LM eds. Nuclear Medicine Annual. 1995, :pp 1-50 Raven Press, New York
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

