Agrin is a major heparan sulfate proteoglycan accumulating in Alzheimer's disease brain
- PMID: 10595940
- PMCID: PMC1866925
- DOI: 10.1016/S0002-9440(10)65529-0
Agrin is a major heparan sulfate proteoglycan accumulating in Alzheimer's disease brain
Abstract
Heparan sulfate proteoglycans (HSPGs) have been suggested to play an important role in the formation and persistence of senile plaques and neurofibrillary tangles in dementia of the Alzheimer's type (DAT). We performed a comparative immunohistochemical analysis of the expression of the HSPGs agrin, perlecan, glypican-1, and syndecans 1-3 in the lesions of DAT brain neocortex and hippocampus. Using a panel of specific antibodies directed against the protein backbone of the various HSPG species and against the glycosaminoglycan (GAG) side-chains, we demonstrated the following. The basement membrane-associated HSPG, agrin, is widely expressed in senile plaques, neurofibrillary tangles and cerebral blood vessels, whereas the expression of the other basement membrane-associated HSPG, perlecan, is lacking in senile plaques and neurofibrillary tangles and is restricted to the cerebral vasculature. Glypican and three different syndecans, all cell membrane-associated HSPG species, are also expressed in senile plaques and neurofibrillary tangles, albeit at a lower frequency than agrin. Heparan sulfate GAG side chains are also associated with both senile plaques and neurofibrillary tangles. Our results suggest that glycosaminoglycan side chains of the HSPGs agrin, syndecan, and glypican, but not perlecan, may play an important role in the formation of both senile plaques and neurofibrillary tangles. In addition, we speculate that agrin, because it contains nine protease-inhibiting domains, may protect the protein aggregates in senile plaques and neurofibrillary tangles against extracellular proteolytic degradation, leading to the persistence of these deposits.
Figures
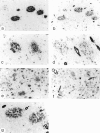
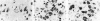





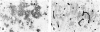
Similar articles
-
Heparan sulfate proteoglycan expression in cerebrovascular amyloid beta deposits in Alzheimer's disease and hereditary cerebral hemorrhage with amyloidosis (Dutch) brains.Acta Neuropathol. 2001 Dec;102(6):604-14. doi: 10.1007/s004010100414. Acta Neuropathol. 2001. PMID: 11761721
-
Heparan sulfate proteoglycan in diffuse plaques of hippocampus but not of cerebellum in Alzheimer's disease brain.Am J Pathol. 1994 Feb;144(2):337-47. Am J Pathol. 1994. PMID: 8311117 Free PMC article.
-
Interaction between beta-amyloid protein and heparan sulfate proteoglycans from the cerebral capillary basement membrane in Alzheimer's disease.J Clin Neurosci. 2009 Feb;16(2):277-82. doi: 10.1016/j.jocn.2008.04.009. Epub 2008 Dec 16. J Clin Neurosci. 2009. PMID: 19091577
-
[The lesions of Alzheimer's disease: which therapeutic perspectives?].Bull Acad Natl Med. 2008 Feb;192(2):303-18; discussion 318-21. Bull Acad Natl Med. 2008. PMID: 18819685 Review. French.
-
Alzheimer's disease and heparan sulfate proteoglycan.Front Biosci. 1998 Mar 21;3:d327-37. doi: 10.2741/a277. Front Biosci. 1998. PMID: 9490646 Review.
Cited by
-
Glycosaminoglycans from Alzheimer's disease hippocampus have altered capacities to bind and regulate growth factors activities and to bind tau.PLoS One. 2019 Jan 4;14(1):e0209573. doi: 10.1371/journal.pone.0209573. eCollection 2019. PLoS One. 2019. PMID: 30608949 Free PMC article.
-
Review of Alterations in Perlecan-Associated Vascular Risk Factors in Dementia.Int J Mol Sci. 2020 Jan 20;21(2):679. doi: 10.3390/ijms21020679. Int J Mol Sci. 2020. PMID: 31968632 Free PMC article. Review.
-
Proteolytic Remodeling of Perineuronal Nets: Effects on Synaptic Plasticity and Neuronal Population Dynamics.Neural Plast. 2018 Feb 4;2018:5735789. doi: 10.1155/2018/5735789. eCollection 2018. Neural Plast. 2018. PMID: 29531525 Free PMC article. Review.
-
Alzheimer's disease is an inherent, natural part of human brain aging: an integrated perspective.Free Neuropathol. 2022 Jul 8;3:17. doi: 10.17879/freeneuropathology-2022-3806. eCollection 2022 Jan. Free Neuropathol. 2022. PMID: 37284149 Free PMC article.
-
Heat shock proteins and amateur chaperones in amyloid-Beta accumulation and clearance in Alzheimer's disease.Mol Neurobiol. 2007 Jun;35(3):203-16. doi: 10.1007/s12035-007-0029-7. Mol Neurobiol. 2007. PMID: 17917109 Free PMC article. Review.
References
-
- Selkoe DJ: The molecular pathology of Alzheimer’s disease. Neuron 1991, 6:487-498 - PubMed
-
- Lee VMY, Balin BJ, Otvos L, Trojanowski JQ: A68: a major subunit of paired helical filaments and derivatized forms of normal tau. Science 1991, 251:675-678 - PubMed
-
- Grundke-Iqbal I, Iqbal K, Quinlan M, Tung Y-C, Zaidi MS, Wisniewski HM: Microtubule-associated protein tau: a component of Alzheimer paired helical filaments. J Biol Chem 1986, 261:6084-6089 - PubMed
-
- Glenner GG, Wong CW: Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun 1984, 120:885-890 - PubMed
-
- Zhan S-S, Veerhuis R, Kamphorst W, Eikelenboom P: Distribution of β amyloid associated proteins in plaques in Alzheimer’s disease and in the non-demented elderly. Neurodegeneration 1995, 4:291-297 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

