Glycoprotein Ib is homogeneously distributed on external and internal membranes of resting platelets
- PMID: 10595941
- PMCID: PMC1866942
- DOI: 10.1016/S0002-9440(10)65530-7
Glycoprotein Ib is homogeneously distributed on external and internal membranes of resting platelets
Abstract
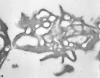
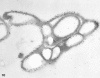
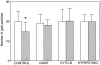
Recent ultrastructural studies have suggested that Glycoprotein Ib (GPIb) has a different distribution on external (surface) versus internal (open canalicular system) membranes in resting discoid platelets. The differential distribution proposed for GPIb differs from that reported for the fibrinogen receptor, GPIIb-IIIa, and could have profound physiological significance when platelets are activated by surfaces. The present study explored the distribution of GPIb on external and internal membranes of resting platelets. Immunogold cytochemical techniques were applied to ultrathin cryosections of washed platelets. Polyclonal antibodies or mixtures of monoclonal antibodies (AP1 and 6D1) were used for labeling. To avoid the technical problem posed by limited accessibility of antigens located in very narrow portions of the open canalicular system (OCS) to antibodies, the same methods were applied to patients with giant platelets syndromes. The OCS of normal resting platelets was also dilated by exposure of platelets to hypertonic conditions or to cytochalasin-B, an agent that prevents assembly of actin, and, reportedly, movement of GPIb. Morphometric analysis revealed that rates of labeling on internal versus external membranes of giant platelets does not differ significantly (0.93 +/- 0.20), provided the OCS is sufficiently dilated. Platelets exposed to cytochalasin B (1.01 +/- 0.31) or to hypertonic conditions (0.96 +/- 0.20) revealed similar ratios for immunogold particles on external and internal membranes. Results of our study indicate that membranes of the exposed surface and lining OCS channels of resting platelets are continuous, identical structures and GPIb is homogeneously distributed on external and internal membranes.
Figures
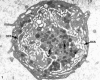
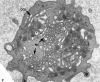
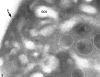
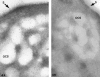
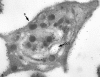
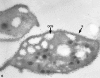
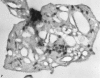
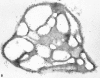
Similar articles
-
Prelabeled glycoprotein Ib/IX receptors are not cleared from exposed surfaces of thrombin-activated platelets.Am J Pathol. 1996 Aug;149(2):629-38. Am J Pathol. 1996. PMID: 8702001 Free PMC article.
-
Fate of the GPIb/IX receptor complex following activation of human platelets.Blood Coagul Fibrinolysis. 1996 Mar;7(2):262-5. doi: 10.1097/00001721-199603000-00039. Blood Coagul Fibrinolysis. 1996. PMID: 8735835
-
Persistence of mobile receptors on surface- and suspension-activated platelets.J Lab Clin Med. 1994 Apr;123(4):536-46. J Lab Clin Med. 1994. PMID: 8145002
-
Differential redistribution of platelet glycoproteins Ib and IIb-IIIa after plasmin stimulation.Blood. 1991 Feb 15;77(4):694-9. Blood. 1991. PMID: 1825180
-
Structure and function of the open canalicular system - the platelet's specialized internal membrane network.Platelets. 2018 Jun;29(4):319-325. doi: 10.1080/09537104.2018.1431388. Epub 2018 Feb 14. Platelets. 2018. PMID: 29442528 Review.
Cited by
-
Platelet-Monocyte Aggregate Instigates Inflammation and Vasculopathy in Kawasaki Disease.Adv Sci (Weinh). 2025 Feb;12(5):e2406282. doi: 10.1002/advs.202406282. Epub 2024 Dec 12. Adv Sci (Weinh). 2025. PMID: 39665236 Free PMC article.
-
Mechanism of platelet adhesion to von Willebrand factor and microparticle formation under high shear stress.Blood. 2006 May 1;107(9):3537-45. doi: 10.1182/blood-2005-02-0618. Epub 2006 Jan 31. Blood. 2006. PMID: 16449527 Free PMC article.
-
Platelets and diseases: signal transduction and advances in targeted therapy.Signal Transduct Target Ther. 2025 May 16;10(1):159. doi: 10.1038/s41392-025-02198-8. Signal Transduct Target Ther. 2025. PMID: 40374650 Free PMC article. Review.
-
The class II PI 3-kinase, PI3KC2α, links platelet internal membrane structure to shear-dependent adhesive function.Nat Commun. 2015 Mar 17;6:6535. doi: 10.1038/ncomms7535. Nat Commun. 2015. PMID: 25779105
-
Aspirin: pharmacology and clinical applications.Thrombosis. 2012;2012:173124. doi: 10.1155/2012/173124. Epub 2011 Nov 17. Thrombosis. 2012. PMID: 22195279 Free PMC article.
References
-
- Tschopp TB, Weiss HJ, Baumgartner HR: Decreased adhesion of platelets to subendothelium in von Willebrand’s disease. J Lab Clin Med 1974, 83:296-300 - PubMed
-
- Bolhuis PA, Sakariassen KS, Sander HJ, Bouma BN, Sixma JJ: Binding of factor VIII-von Willebrand factor to human arterial subendothelium precedes increased platelet adhesion and enhances platelet spreading. J Lab Clin Med 1981, 97:568-576 - PubMed
-
- Turitto VT, Weiss HJ, Baumgartner HR: Decreased platelet adhesion on vessel segments in von Willebrand’s disease: a defect in initial platelet attachment. J Lab Clin Med 1983, 102:551-564 - PubMed
-
- Weiss HJ, Turitto VT, Baumgartner HR: Platelet adhesion and thrombus formation on subendothelium in platelets deficient in glycoproteins IIb-IIIa, Ib, and storage granules. Blood 1986, 67:322-330 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

