Interleukin-1 and tumor necrosis factor receptor signaling is not required for bacteria-induced osteoclastogenesis and bone loss but is essential for protecting the host from a mixed anaerobic infection
- PMID: 10595943
- PMCID: PMC1866914
- DOI: 10.1016/S0002-9440(10)65532-0
Interleukin-1 and tumor necrosis factor receptor signaling is not required for bacteria-induced osteoclastogenesis and bone loss but is essential for protecting the host from a mixed anaerobic infection
Abstract
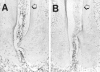
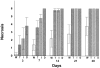
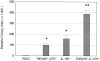
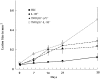
Bacterial infection causes significant morbidity, mediated in part by the up-regulation of inflammatory cytokines. Cytokine induction is thought to stimulate osteolysis in conditions such as periodontal disease and otitis media. To establish the relative importance of interleukin-1 (IL-1) and tumor necrosis factor (TNF) in mediating the response to a mixed anaerobic infection, we used an in vivo model in which the dental pulp was inoculated with six anaerobic pathogens, in mice with functional deletions of receptors to IL-1 (IL-1RI(-/-)), TNF (TNFRp55(-/-)-p75(-/-)), or both (TNFRp55(-/-)-IL-1RI(-/-)). Polymorphonuclear and mononuclear phagocyte recruitment occurred to the greatest extent in TNFRp55(-/-)-IL-1RI(-/-) mice, and to a lesser extent in IL-1RI(-/-) or TNFRp55(-/-)-p75(-/-) mice, and the least in wild-type mice, demonstrating that recruitment of these phagocytes is not dependent on IL-1 or TNF receptor signaling. A similar pattern was observed for bacterial penetration into host tissue. Because it had recently been reported that TNF played a critical role in mediating lipopolysaccharide-induced bone loss, we anticipated that mice with targeted deletions of TNFRp55(-/-) would have reduced osteoclastogenesis. Surprisingly, osteolytic lesion formation was greatest in animals lacking TNF and/or IL-1 receptors. These results indicate that IL-1 or TNF receptor signaling is not required for bacteria-induced osteoclastogenesis and bone loss, but does play a critical role in protecting the host against mixed anaerobic infections.
Figures

Similar articles
-
Interleukin-1 receptor signaling rather than that of tumor necrosis factor is critical in protecting the host from the severe consequences of a polymicrobe anaerobic infection.Infect Immun. 2000 Aug;68(8):4746-51. doi: 10.1128/IAI.68.8.4746-4751.2000. Infect Immun. 2000. PMID: 10899881 Free PMC article.
-
Contribution of interleukin-11 and prostaglandin(s) in lipopolysaccharide-induced bone resorption in vivo.Infect Immun. 2002 Jul;70(7):3915-22. doi: 10.1128/IAI.70.7.3915-3922.2002. Infect Immun. 2002. PMID: 12065535 Free PMC article.
-
IL-1 mediates TNF-induced osteoclastogenesis.J Clin Invest. 2005 Feb;115(2):282-90. doi: 10.1172/JCI23394. J Clin Invest. 2005. PMID: 15668736 Free PMC article.
-
TNF-alpha regulates corneal Langerhans cell migration.J Immunol. 1999 Apr 1;162(7):4235-9. J Immunol. 1999. PMID: 10201952
-
Combining anaerobic bacterial oncolysis with vaccination that blocks interleukin-10 signaling may achieve better outcomes for late stage cancer management.Hum Vaccin Immunother. 2016 Mar 3;12(3):599-606. doi: 10.1080/21645515.2015.1089008. Hum Vaccin Immunother. 2016. PMID: 26367244 Free PMC article. Review.
Cited by
-
Developing animal models for polymicrobial diseases.Nat Rev Microbiol. 2004 Jul;2(7):552-68. doi: 10.1038/nrmicro928. Nat Rev Microbiol. 2004. PMID: 15197391 Free PMC article. Review.
-
Rapid linkage of innate immunological signals to adaptive immunity by the brain-fat axis.Nat Immunol. 2015 May;16(5):525-33. doi: 10.1038/ni.3133. Epub 2015 Apr 6. Nat Immunol. 2015. PMID: 25848866 Free PMC article.
-
Sleep-wake behavior and responses to sleep deprivation of mice lacking both interleukin-1 beta receptor 1 and tumor necrosis factor-alpha receptor 1.Brain Behav Immun. 2008 Aug;22(6):982-93. doi: 10.1016/j.bbi.2008.02.001. Epub 2008 Mar 7. Brain Behav Immun. 2008. PMID: 18329246 Free PMC article.
-
Tumor necrosis factor-alpha and interleukin 6 in human periapical lesions.Mediators Inflamm. 2007;2007:38210. doi: 10.1155/2007/38210. Epub 2006 Dec 27. Mediators Inflamm. 2007. PMID: 17497030 Free PMC article.
-
Inflammation and uncoupling as mechanisms of periodontal bone loss.J Dent Res. 2011 Feb;90(2):143-53. doi: 10.1177/0022034510385236. Epub 2010 Dec 6. J Dent Res. 2011. PMID: 21135192 Free PMC article.
References
-
- Birkedal-Hansen H: Role of cytokines and inflammatory mediators in tissue destruction. J Periodontal Res 1993, 28:500-510 - PubMed
-
- Reddy SV, Roodman GD: Control of osteoclast differentiation. Crit Rev Eukaryot Gene Expr 1998, 8:1-17 - PubMed
-
- Boyce BF, Hughes DE, Wright KR, Xing L, Dai A: Recent advances in bone biology provide insight into the pathogenesis of bone diseases. Lab Invest 1999, 79:83-94 - PubMed
-
- Dinarello CA: Biologic basis for interleukin-1 in disease. Blood 1996, 87:2095-2147 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

