Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development
- PMID: 10601040
- PMCID: PMC317181
- DOI: 10.1101/gad.13.23.3149
Wnt signaling in Xenopus embryos inhibits bmp4 expression and activates neural development
Abstract
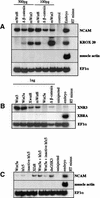
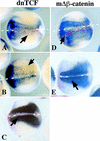
We report a new role for Wnt signaling in the vertebrate embryo: the induction of neural tissue from ectoderm. Early expression of mouse wnt8, Xwnt8, beta-catenin, or dominant-negative GSK3 induces the expression of neural-specific markers and inhibits the expression of Bmp4 in Xenopus ectoderm. We show that Wnt8, but not the BMP antagonist Noggin, can inhibit Bmp4 expression at early gastrula stages. Furthermore, inhibition of beta-catenin activity in the neural ectoderm of whole embryos by a truncated TCF results in a decrease in neural development. Therefore, we suggest that a cleavage-stage Wnt signal normally contributes to an early repression of Bmp4 on the dorsal side of the embryo and sensitizes the ectoderm to respond to neural inducing signals from the organizer. The Wnt targets Xnr3 and siamois have been shown previously to have neuralizing activity when overexpressed. However, antagonists of Wnt signaling, dnXwnt8 and Nxfrz8, inhibit Wnt-mediated Xnr3 and siamois induction, but not neural induction, suggesting an alternative mechanism for Bmp repression and neuralization. Conversely, dnTCF blocks both Wnt-mediated Xnr3 and neural induction, suggesting that both pathways require this transcription factor.
Figures







References
-
- Baker JC, Harland RM. A novel mesoderm inducer, Madr2, functions in the activin signal transduction pathway. Genes & Dev. 1996;10:1880–1889. - PubMed
-
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neutralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. - PubMed
-
- Bhanot P, Brink M, Samos CH, Hsieh JC, Wang Y, Macke JP, Andrew D, Nathans J, Nusse R. A new member of the frizzled family from Drosophila functions as a Wingless receptor. Nature. 1996;382:225–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
