Localization of synthesis of beta1,6-glucan in Saccharomyces cerevisiae
- PMID: 10601196
- PMCID: PMC94196
- DOI: 10.1128/JB.181.24.7414-7420.1999
Localization of synthesis of beta1,6-glucan in Saccharomyces cerevisiae
Abstract
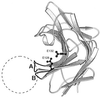
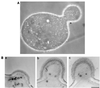
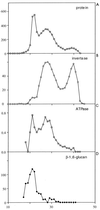
Beta1,6-Glucan is a key component of the yeast cell wall, interconnecting cell wall proteins, beta1,3-glucan, and chitin. It has been postulated that the synthesis of beta1,6-glucan begins in the endoplasmic reticulum with the formation of protein-bound primer structures and that these primer structures are extended in the Golgi complex by two putative glucosyltransferases that are functionally redundant, Kre6 and Skn1. This is followed by maturation steps at the cell surface and by coupling to other cell wall macromolecules. We have reinvestigated the role of Kre6 and Skn1 in the biogenesis of beta1,6-glucan. Using hydrophobic cluster analysis, we found that Kre6 and Skn1 show significant similarities to family 16 glycoside hydrolases but not to nucleotide diphospho-sugar glycosyltransferases, indicating that they are glucosyl hydrolases or transglucosylases instead of genuine glucosyltransferases. Next, using immunogold labeling, we tried to visualize intracellular beta1,6-glucan in cryofixed sec1-1 cells which had accumulated secretory vesicles at the restrictive temperature. No intracellular labeling was observed, but the cell surface was heavily labeled. Consistent with this, we could detect substantial amounts of beta1,6-glucan in isolated plasma membrane-derived microsomes but not in post-Golgi secretory vesicles. Taken together, our data indicate that the synthesis of beta1, 6-glucan takes place largely at the cell surface. An alternative function for Kre6 and Skn1 is discussed.
Figures


References
-
- Barbeyron T, Gerard A, Potin P, Henrissat B, Kloareg B. The kappa-carrageenase of the marine bacterium Cytophaga drobachiensis. Structural and phylogenetic relationships within family-16 glycoside hydrolases. Mol Biol Evol. 1998;15:528–537. - PubMed
-
- Bowman B J, Slayman C W. The effect of vanadate on the plasma membrane ATPase of Neurospora crassa. J Biol Chem. 1979;254:2928–2934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

