The A-kinase-anchoring protein AKAP95 is a multivalent protein with a key role in chromatin condensation at mitosis
- PMID: 10601332
- PMCID: PMC2168084
- DOI: 10.1083/jcb.147.6.1167
The A-kinase-anchoring protein AKAP95 is a multivalent protein with a key role in chromatin condensation at mitosis
Abstract
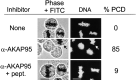
Protein kinase A (PKA) and the nuclear A-kinase-anchoring protein AKAP95 have previously been shown to localize in separate compartments in interphase but associate at mitosis. We demonstrate here a role for the mitotic AKAP95-PKA complex. In HeLa cells, AKAP95 is associated with the nuclear matrix in interphase and redistributes mostly into a chromatin fraction at mitosis. In a cytosolic extract derived from mitotic cells, AKAP95 recruits the RIIalpha regulatory subunit of PKA onto chromatin. Intranuclear immunoblocking of AKAP95 inhibits chromosome condensation at mitosis and in mitotic extract in a PKA-independent manner. Immunodepletion of AKAP95 from the extract or immunoblocking of AKAP95 at metaphase induces premature chromatin decondensation. Condensation is restored in vitro by a recombinant AKAP95 fragment comprising the 306-carboxy-terminal amino acids of the protein. Maintenance of condensed chromatin requires PKA binding to chromatin-associated AKAP95 and cAMP signaling through PKA. Chromatin-associated AKAP95 interacts with Eg7, the human homologue of Xenopus pEg7, a component of the 13S condensin complex. Moreover, immunoblocking nuclear AKAP95 inhibits the recruitment of Eg7 to chromatin in vitro. We propose that AKAP95 is a multivalent molecule that in addition to anchoring a cAMP/PKA-signaling complex onto chromosomes, plays a role in regulating chromosome structure at mitosis.
Figures
















References
-
- Adachi Y., Luke M., Laemmli U.K. Chromosome assembly in vitrotopoisomerase II is required for condensation. Cell. 1991;64:137–148. - PubMed
-
- Buendia B., Courvalin J.-C. Domain-specific disassembly and reassembly of nuclear membranes during mitosis. Exp. Cell Res. 1997;230:133–144. - PubMed
-
- Carr D.W., Stofko-Hahn R.E., Fraser I.D., Bishop S.M., Acott T.S., Brennan R.G., Scott J.D. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J. Biol. Chem. 1991;266:14188–14192. - PubMed
-
- Coghlan V.M., Langeberg L.K., Fernandez A., Lamb N.J., Scott J.D. Cloning and characterization of AKAP 95, a nuclear protein that associates with the regulatory subunit of type II cAMP-dependent protein kinase. J. Biol. Chem. 1994;269:7658–7665. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

