ER to Golgi transport: Requirement for p115 at a pre-Golgi VTC stage
- PMID: 10601335
- PMCID: PMC2168100
- DOI: 10.1083/jcb.147.6.1205
ER to Golgi transport: Requirement for p115 at a pre-Golgi VTC stage
Abstract
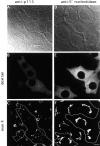
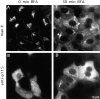
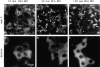
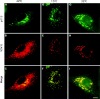
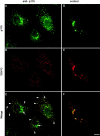
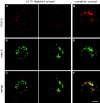
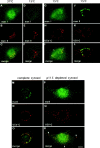
The membrane transport factor p115 functions in the secretory pathway of mammalian cells. Using biochemical and morphological approaches, we show that p115 participates in the assembly and maintenance of normal Golgi structure and is required for ER to Golgi traffic at a pre-Golgi stage. Injection of antibodies against p115 into intact WIF-B cells caused Golgi disruption and inhibited Golgi complex reassembly after BFA treatment and wash-out. Addition of anti-p115 antibodies or depletion of p115 from a VSVtsO45 based semi-intact cell transport assay inhibited transport. The inhibition occurred after VSV glycoprotein (VSV-G) exit from the ER but before its delivery to the Golgi complex, and resulted in VSV-G protein accumulating in peripheral vesicular tubular clusters (VTCs). The p115-requiring step of transport followed the rab1-requiring step and preceded the Ca(2+)-requiring step. Unexpectedly, mannosidase I redistributed from the Golgi complex to colocalize with VSV-G protein arrested in pre-Golgi VTCs by p115 depletion. Redistribution of mannosidase I was also observed in cells incubated at 15 degrees C. Our data show that p115 is essential for the translocation of pre-Golgi VTCs from peripheral sites to the Golgi stack. This defines a previously uncharacterized function for p115 at the VTC stage of ER to Golgi traffic.
Figures


References
-
- Balch W.E., Elliott M.M., Keller D.S. ATP-coupled transport of vesicular stomatitis virus G protein between the endoplasmic reticulum and the Golgi. J. Biol. Chem. 1986;261:14681–14689. - PubMed
-
- Balch W.E., McCaffery J.M., Plutner H., Farquhar M.G. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

