Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function
- PMID: 10601336
- PMCID: PMC2168089
- DOI: 10.1083/jcb.147.6.1223
Role for Drs2p, a P-type ATPase and potential aminophospholipid translocase, in yeast late Golgi function
Abstract
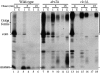
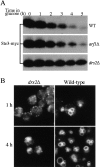
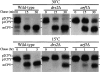
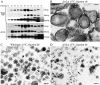
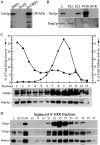
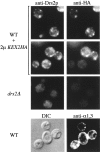
ADP-ribosylation factor appears to regulate the budding of both COPI and clathrin-coated transport vesicles from Golgi membranes. An arf1Delta synthetic lethal screen identified SWA3/DRS2, which encodes an integral membrane P-type ATPase and potential aminophospholipid translocase (or flippase). The drs2 null allele is also synthetically lethal with clathrin heavy chain (chc1) temperature-sensitive alleles, but not with mutations in COPI subunits or other SEC genes tested. Consistent with these genetic analyses, we found that the drs2Delta mutant exhibits late Golgi defects that may result from a loss of clathrin function at this compartment. These include a defect in the Kex2-dependent processing of pro-alpha-factor and the accumulation of abnormal Golgi cisternae. Moreover, we observed a marked reduction in clathrin-coated vesicles that can be isolated from the drs2Delta cells. Subcellular fractionation and immunofluorescence analysis indicate that Drs2p localizes to late Golgi membranes containing Kex2p. These observations indicate a novel role for a P-type ATPase in late Golgi function and suggest a possible link between membrane asymmetry and clathrin function at the Golgi complex.
Figures


References
-
- Allen G., Green N.M. A 31-residue tryptic peptide from the active site of the [Ca++]-transporting ATPase of rabbit sarcoplasmic reticulum. FEBS Lett. 1976;63:188–192. - PubMed
-
- Barik S., Galinski M.S. Megaprimer method of PCRincreased template concentration improves yield. Biotechnology. 1991;10:489–490. - PubMed
-
- Catty P., de Kerchove d'Exaerde A., Goffeau A. The complete inventory of the yeast Saccharomyces cerevisiae P-type transport ATPases. FEBS Lett. 1997;409:325–332. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

