Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration
- PMID: 10601352
- PMCID: PMC2195714
- DOI: 10.1084/jem.190.12.1769
Targeted gene disruption demonstrates that P-selectin glycoprotein ligand 1 (PSGL-1) is required for P-selectin-mediated but not E-selectin-mediated neutrophil rolling and migration
Abstract
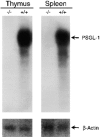
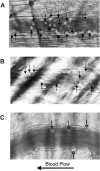
P-selectin glycoprotein ligand 1 (PSGL-1) is a mucin-like selectin counterreceptor that binds to P-selectin, E-selectin, and L-selectin. To determine its physiological role in cell adhesion as a mediator of leukocyte rolling and migration during inflammation, we prepared mice genetically deficient in PSGL-1 by targeted disruption of the PSGL-1 gene. The homozygous PSGL-1-deficient mouse was viable and fertile. The blood neutrophil count was modestly elevated. There was no evidence of spontaneous development of skin ulcerations or infections. Leukocyte infiltration in the chemical peritonitis model was significantly delayed. Leukocyte rolling in vivo, studied by intravital microscopy in postcapillary venules of the cremaster muscle, was markedly decreased 30 min after trauma in the PSGL-1-deficient mouse. In contrast, leukocyte rolling 2 h after tumor necrosis factor alpha stimulation was only modestly reduced, but blocking antibodies to E-selectin infused into the PSGL-1-deficient mouse almost completely eliminated leukocyte rolling. These results indicate that PSGL-1 is required for the early inflammatory responses but not for E-selectin-mediated responses. These kinetics are consistent with a model in which PSGL-1 is the predominant neutrophil P-selectin ligand but is not a required counterreceptor for E-selectin under in vivo physiological conditions.
Figures








Similar articles
-
E-selectin engages PSGL-1 and CD44 through a common signaling pathway to induce integrin alphaLbeta2-mediated slow leukocyte rolling.Blood. 2010 Jul 22;116(3):485-94. doi: 10.1182/blood-2009-12-259556. Epub 2010 Mar 18. Blood. 2010. PMID: 20299514 Free PMC article.
-
P-selectin glycoprotein ligand-1 mediates L-selectin-dependent leukocyte rolling in venules.J Exp Med. 2003 May 19;197(10):1355-63. doi: 10.1084/jem.20021854. J Exp Med. 2003. PMID: 12756271 Free PMC article.
-
An L-selectin ligand distinct from P-selectin glycoprotein ligand-1 is expressed on endothelial cells and promotes neutrophil rolling in inflammation.Blood. 2008 Dec 15;112(13):4915-23. doi: 10.1182/blood-2008-04-153866. Epub 2008 Sep 25. Blood. 2008. PMID: 18818390 Free PMC article.
-
Structure and function of P-selectin glycoprotein ligand-1.Leuk Lymphoma. 1998 Mar;29(1-2):1-15. doi: 10.3109/10428199809058377. Leuk Lymphoma. 1998. PMID: 9638971 Review.
-
The biology of P-selectin glycoprotein ligand-1: its role as a selectin counterreceptor in leukocyte-endothelial and leukocyte-platelet interaction.Thromb Haemost. 1999 Jan;81(1):1-7. Thromb Haemost. 1999. PMID: 10348699 Review.
Cited by
-
CD44 is a physiological E-selectin ligand on neutrophils.J Exp Med. 2005 Apr 18;201(8):1183-9. doi: 10.1084/jem.20042014. Epub 2005 Apr 11. J Exp Med. 2005. PMID: 15824084 Free PMC article.
-
P-selectin glycoprotein ligand-1 deficiency is protective against obesity-related insulin resistance.Diabetes. 2011 Jan;60(1):189-99. doi: 10.2337/db09-1894. Epub 2010 Oct 22. Diabetes. 2011. PMID: 20971965 Free PMC article.
-
A semianalytical model to study the effect of cortical tension on cell rolling.Biophys J. 2010 Dec 15;99(12):3870-9. doi: 10.1016/j.bpj.2010.10.038. Biophys J. 2010. PMID: 21156128 Free PMC article.
-
Innate Immune Basis for Rift Valley Fever Susceptibility in Mouse Models.Sci Rep. 2017 Aug 2;7(1):7096. doi: 10.1038/s41598-017-07543-8. Sci Rep. 2017. PMID: 28769107 Free PMC article.
-
Soluble Siglec-5 associates to PSGL-1 and displays anti-inflammatory activity.Sci Rep. 2016 Nov 28;6:37953. doi: 10.1038/srep37953. Sci Rep. 2016. PMID: 27892504 Free PMC article.
References
-
- Springer T.A. Traffic signals for lymphocyte recirculation and leukocyte emigrationthe multistep paradigm. Cell. 1994;76:301–314. - PubMed
-
- Tedder T.F., Steeber D.A., Chen A., Engel P. The selectinsvascular adhesion molecules. FASEB J. 1995;9:866–873. - PubMed
-
- McEver R.P., Moore K.L., Cummings R.D. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J. Biol. Chem. 1995;270:11025–11028. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

