Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells
- PMID: 10601362
- PMCID: PMC2195712
- DOI: 10.1084/jem.190.12.1879
Early activation of caspases during T lymphocyte stimulation results in selective substrate cleavage in nonapoptotic cells
Abstract
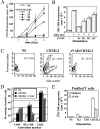
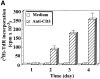
Apoptosis induced by T cell receptor (TCR) triggering in T lymphocytes involves activation of cysteine proteases of the caspase family through their proteolytic processing. Caspase-3 cleavage was also reported during T cell stimulation in the absence of apoptosis, although the physiological relevance of this response remains unclear. We show here that the caspase inhibitor benzyloxycarbonyl (Cbz)-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD) blocks proliferation, major histocompatibility complex class II expression, and blastic transformation during stimulation of peripheral blood lymphocytes. Moreover, T cell activation triggers the selective processing and activation of downstream caspases (caspase-3, -6, and -7), but not caspase-1, -2, or -4, as demonstrated even in intact cells using a cell-permeable fluorescent substrate. Caspase-3 processing occurs in different T cell subsets (CD4(+), CD8(+), CD45RA(+), and CD45RO(+)), and in activated B lymphocytes. The pathway leading to caspase activation involves death receptors and caspase-8, which is also processed after TCR triggering, but not caspase-9, which remains as a proenzyme. Most importantly, caspase activity results in a selective substrate specificity, since poly(ADP-ribose) polymerase (PARP), lamin B, and Wee1 kinase, but not DNA fragmentation factor (DFF45) or replication factor C (RFC140), are processed. Caspase and substrate processing occur in nonapoptotic lymphocytes. Thus, caspase activation is an early and physiological response in viable, stimulated lymphocytes, and appears to be involved in early steps of lymphocyte activation.
Figures




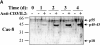
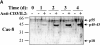








Comment in
-
Caspases. Multifunctional proteases.J Exp Med. 1999 Dec 20;190(12):1725-8. doi: 10.1084/jem.190.12.1725. J Exp Med. 1999. PMID: 10601347 Free PMC article. No abstract available.
References
-
- Thornberry N.A., Lazebnik Y. Caspasesenemies within. Science. 1998;281:1312–1316. - PubMed
-
- Nicholson D.W., Ali A., Thornberry N.A., Vaillancourt J.P., Ding C.K., Gallant M., Gareau Y., Griffin P.R., Labelle M., Lazebnick Y.A. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature. 1995;376:37–43. - PubMed
-
- Han Z., Hendrickson E.A., Bremner T.A., Wyche J.H. A sequential two-step mechanism for the production of the mature P17:P12 form of caspase-3 in vitro . J. Biol. Chem. 1997;272:13432–13436. - PubMed
-
- Clayton L.K., Ghendler Y., Mizoguchi E., Patch R.J., Ocain T.D., Orth K., Bhan A.K., Dixit V.M., Reinherz E.L. T-cell receptor ligation by peptide/MHC induces activation of a caspase in immature thymocytesthe molecular basis of negative selection. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:2282–2293. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

