Activation of muscle nicotinic acetylcholine receptor channels by nicotinic and muscarinic agonists
- PMID: 10602325
- PMCID: PMC1571784
- DOI: 10.1038/sj.bjp.0702941
Activation of muscle nicotinic acetylcholine receptor channels by nicotinic and muscarinic agonists
Abstract
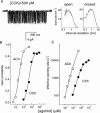
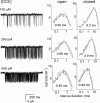
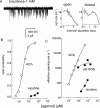
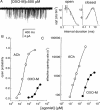
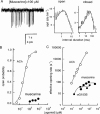
1. The dose-response parameters of recombinant mouse adult neuromuscular acetylcholine receptor channels (nAChR) activated by carbamylcholine, nicotine, muscarine and oxotremorine were measured. Rate constants for agonist association and dissociation, and channel opening and closing, were estimated from single-channel kinetic analysis. 2. The dissociation equilibrium constants were (mM): ACh (0. 16)<oxotremorine M (0.6)<carbamylcholine (0.8)<nicotine (2.6). 3. The gating equilibrium constants (opening/closing) were: ACh (45)>carbamylcholine (5.1)>oxotremorine M (0.6)>nicotine (0. 5)>muscarine (0.15). 4. Rat neuronal alpha4beta2 nAChR can be activated by all of the agonists. However, detailed kinetic analysis was impossible because the recordings lacked clusters representing the activity of a single receptor complex. Thus, the number of channels in the patch was unknown and the activation rate constants could not be determined. 5. Considering both receptor affinity and agonist efficacy, muscarine and oxotremorine are significant agonists of muscle-type nAChR. The results are discussed in terms of structure-function relationships at the nAChR transmitter binding site.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

