Modulation of mPer1 gene expression by anxiolytic drugs in mouse cerebellum
- PMID: 10602344
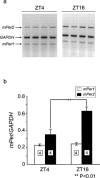
- PMCID: PMC1571793
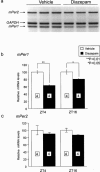
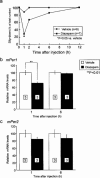
- DOI: 10.1038/sj.bjp.0702957
Modulation of mPer1 gene expression by anxiolytic drugs in mouse cerebellum
Abstract
1. The mPer1 and mPer2 genes are putative mouse clock genes that regulate circadian oscillator present in the suprachiasmatic nucleus (SCN) neuron. While they are also expressed in the granular cell layer in the cerebellum, their function is unknown. In a first step to verify the physiological roles of mPer1 and mPer2 genes in the cerebellum, we examined the effects of benzodiazepines on the expression of the mPer1 and mPer2 genes. 2. mPer2 mRNA expression was higher at ZT16 than ZT4 in the mouse cerebellum. 3. High-dose administration of diazepam (10 mg kg-1) or triazolam (1 mg kg-1) reduced mPer1 mRNA level 1 h after treatment in the cerebellum. 4. Reduced expression of mPer1 by diazepam treatment was transient. No difference of mPer1 mRNA level between diazepam (10 mg kg-1)- and vehicle-treated group was observed 6 h after treatment. 5. Administration of high doses of tandospirone (30 mg kg-1), a non-benzodiazepine anxiolytic also reduced mPer1 mRNA expression 1 h after treatment. 6. Administration of high doses of clozapine (5 mg kg-1) or haloperidol (1 mg kg-1) impaired the rota-rod performance without affecting on mPer1 mRNA level. 7. Diazepam and tandospirone inhibited the expression of mPer1 mRNA in the primary cultured cerebellum granule cells. 8. Transient reductions of mPer1 mRNA levels by various benzodiazepines and tandospirone is associated with impairment of coordinated movement, such as rota-rod performance and equilibrium.
Figures


References
-
- AIBA A., KANO M., CHEN C., STANTON M.E., FOX G.D., HERRUP K., ZWINGMAN T.A., TONEGAWA S. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79:377–388. - PubMed
-
- AKIYAMA M., KOUZU Y., TAKAHASHI S., WAKAMATSU H., MORIYA T., MAETANI M., WATANABE S., TEI H., SAKAKI Y., SHIBATA S. Inhibition of light- or glutamate-induced mPer1 expression represses the phase shifts into the mouse circadian locomotor and suprachiasmatic firing rhythms. J. Neurosci. 1999;19:1115–1121. - PMC - PubMed
-
- ALBRECHT U., SUN Z.S., EICHELE G., LEE C.C. A differential response of two putative mammalian circadian regulators, mPer1 and mPer2, to light. Cell. 1997;91:1055–1064. - PubMed
-
- ALLADA R., WHITE N.E., SO W.V., HALL J.C., ROSBASH M. A mutant Drosophila homologue of mammalian Clock disrupts circadian rhythms and transcription of period and timeless. Cell. 1998;93:791–804. - PubMed
-
- BALSALOBRE A., DAMIOLA F., SCHIBLER U. A serum shock induces circadian gene expression in mammalian tissue culture cells. Cell. 1998;93:929–937. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

