Zanamivir susceptibility monitoring and characterization of influenza virus clinical isolates obtained during phase II clinical efficacy studies
- PMID: 10602727
- PMCID: PMC89632
- DOI: 10.1128/AAC.44.1.78-87.2000
Zanamivir susceptibility monitoring and characterization of influenza virus clinical isolates obtained during phase II clinical efficacy studies
Abstract
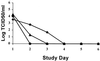
Zanamivir is a highly selective neuraminidase (NA) inhibitor with demonstrated clinical efficacy against influenza A and B virus infections. In phase II clinical efficacy trials (NAIB2005 and NAIB2008), virological substudies showed mean reductions in virus shedding after 24 h of treatment of 1.5 to 2.0 log(10) 50% tissue culture infective doses compared to a placebo, with no reemergence of virus after the completion of therapy. Paired isolates (n = 41) obtained before and during therapy with zanamivir demonstrated no shifts in susceptibility to zanamivir when measured by NA assays, although for a few isolates NA activity was too low to evaluate. In plaque reduction assays in MDCK cells, the susceptibility of isolates to zanamivir was extremely variable even at baseline and did not correlate with the speed of resolution of virus shedding. Isolates with apparent limited susceptibility to zanamivir by plaque reduction proved highly susceptible in vivo in the ferret model. Further sequence analysis of paired isolates revealed no changes in the hemagglutinin and NA genes in the majority of isolates. The few changes observed were all natural variants. No amino acid changes that had previously been identified in vitro as being involved with reduced susceptibility to zanamivir were observed. These studies highlighted problems associated with monitoring susceptibility to NA inhibitors in the clinic, in that no reliable cell-based assay is available. At present the NA assay is the best available predictor of susceptibility to NA inhibitors in vivo, as measured in the validated ferret model of infection.
Figures

References
-
- Blick T J, Sahasrabudhe A, McDonald M, Owens I J, Morley P J, Fenton R J, McKimm-Breschkin J L. The interaction of neuraminidase and hemagglutinin mutations in influenza virus in resistance to 4-guanidino-Neu5Ac2en. Virology. 1998;246:95–103. - PubMed
-
- Blick T J, Tiong T, Sahasrabudhe A, Varghese J N, Colman P M, Hart G J, Bethell R C, McKimm-Breschkin J L. Generation and characterization of an influenza virus neuraminidase variant with decreased susceptibility to the neuraminidase-specific inhibitor 4-guanidino-Neu5Ac2en. Virology. 1995;214:475–484. - PubMed
-
- Campion K, Silagy C, Cooper C, Bolton P, Watts R, Liaw T, Narayan K, Delooze F the MIST Study Group. Efficacy and safety of inhaled zanamivir in the treatment of influenza A and B virus infections. Lancet. 1998;352:1877–1881. - PubMed
-
- Colacino J M, Chirgadze N Y, Garman E, Murti K G, Loncharich R J, Baxter A J, Staschke K A, Laver W G. A single sequence change destabilizes the influenza virus neuraminidase tetramer. Virology. 1997;236:66–75. - PubMed
-
- Doherty R M. Animal virus titration techniques. In: Harris R J C, editor. Techniques in experimental virology. New York, N.Y: Academic Press; 1964. pp. 169–223.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

