Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin
- PMID: 10602736
- PMCID: PMC89641
- DOI: 10.1128/AAC.44.1.143-149.2000
Immunosuppressive and nonimmunosuppressive cyclosporine analogs are toxic to the opportunistic fungal pathogen Cryptococcus neoformans via cyclophilin-dependent inhibition of calcineurin
Abstract
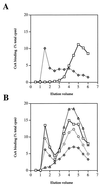
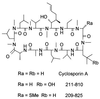
Cyclosporine (CsA) is an immunosuppressive and antimicrobial drug which, in complex with cyclophilin A, inhibits the protein phosphatase calcineurin. We recently found that Cryptococcus neoformans growth is resistant to CsA at 24 degrees C but sensitive at 37 degrees C and that calcineurin is required for growth at 37 degrees C and pathogenicity. Here CsA analogs were screened for toxicity against C. neoformans in vitro. In most cases, antifungal activity was correlated with cyclophilin A binding in vitro and inhibition of the mixed-lymphocyte reaction and interleukin 2 production in cell culture. Two unusual nonimmunosuppressive CsA derivatives, (gamma-OH) MeLeu(4)-Cs (211-810) and D-Sar (alpha-SMe)(3) Val(2)-DH-Cs (209-825), which are also toxic to C. neoformans were identified. These CsA analogs inhibit C. neoformans via fungal cyclophilin A and calcineurin homologs. Our findings identify calcineurin as a novel antifungal drug target and suggest nonimmunosuppressive CsA analogs warrant investigation as antifungal agents.
Figures


References
-
- Baumann G, Andersen E, Quesniaux V, Eberle M K. Cyclosporine and its analogue SDZ IMM 125 mediate very similar effects on T-cell activation—a comparative analysis in vitro. Transplant Proc. 1992;24:43–48. - PubMed
-
- Cameron M L, Schell W A, Bruch S, Bartlett J, Waskin H A, Perfect J R. Correlation of in vitro fluconazole resistance of Candida isolates in relation to therapy and symptoms of individuals seropositive for human immunodeficiency virus type 1. Antimicrob Agents Chemother. 1993;37:2449–2453. - PMC - PubMed
-
- Cardenas M E, Lim E, Heitman J. Mutations that perturb cyclophilin A ligand binding pocket confer cyclosporin A resistance in Saccharomyces cerevisiae. J Biol Chem. 1995;270:20997–21002. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

