Activities of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts
- PMID: 10602737
- PMCID: PMC89642
- DOI: 10.1128/AAC.44.1.150-155.2000
Activities of the triazole derivative SCH 56592 (posaconazole) against drug-resistant strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi in immunocompetent and immunosuppressed murine hosts
Abstract
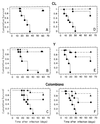
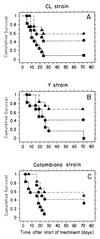
We have studied the in vivo activity of the new experimental triazole derivative SCH 56592 (posaconazole) against a variety of strains of the protozoan parasite Trypanosoma (Schizotrypanum) cruzi, the causative agent of Chagas' disease, in both immunocompetent and immunosuppressed murine hosts. The T. cruzi strains used in the study were previously characterized as susceptible (CL), partially resistant (Y), or highly resistant (Colombiana, SC-28, and VL-10) to the drugs currently in clinical use, nifurtimox and benznidazole. Furthermore, all strains are completely resistant to conventional antifungal azoles, such as ketoconazole. In the first study, acute infections with the CL, Y, and Colombiana strains in both normal and cyclophosphamide-immunosuppressed mice were treated orally, starting 4 days postinfection (p.i.), for 20 consecutive daily doses. The results indicated that in immunocompetent animals SCH 56592 at 20 mg/kg of body weight/day provided protection (80 to 90%) against death caused by all strains, a level comparable or superior to that provided by the optimal dose of benznidazole (100 mg/kg/day). Evaluation of parasitological cure revealed that SCH 56592 was able to cure 90 to 100% of the surviving animals infected with the CL and Y strains and 50% of those which received the benznidazole- and nifurtimox-resistant Colombiana strain. Immunosuppression markedly reduced the mean survival time of untreated mice infected with any of the strains, but this was not observed for the groups which received SCH 56592 at 20 mg/kg/day or benznidazole at 100 mg/kg/day. However, the overall cure rates were higher for animals treated with SCH 56592 than among those treated with benznidazole. The results were confirmed in a second study, using the same model but a longer (43-dose) treatment period. Finally, a model for the chronic disease in which oral treatment was started 120 days p.i. and consisted of 20 daily consecutive doses was investigated. The results showed that SCH 56592 at 20 mg/kg/day was able to induce a statistically significant increase in survival of animals infected with all strains, while benznidazole at 100 mg/kg/day was able to increase survival only in animals infected with the Colombiana strain. Moreover, the triazole was able to induce parasitological cures in 50 to 60% of surviving animals, irrespective of the infecting strain, while no cures were obtained with benznidazole. Taken together, the results demonstrate that SCH 56592 has in vivo trypanocidal activity, even against T. cruzi strains naturally resistant to nitrofurans, nitroimidazoles, and conventional antifungal azoles, and that this activity is retained to a large extent in immunosuppressed hosts.
Figures
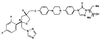
References
-
- Araújo M S S, Molina J, Pereira M E S, Brener Z. Combination of drugs in the treatment of experimental Trypanosoma cruzi infection. Mem Inst Oswaldo Cruz. 1996;91(Suppl. 1):315. - PubMed
-
- Brener Z. Trypanosoma cruzi: taxonomy, morphology and life cycle. In: Wendel S, Brener Z, Camargo M E, Rassi A, editors. Chagas disease (American trypanosomiasis): its impact on tranfusion and clinical medicine. São Paulo, Brazil: ISBT Brazil'92; 1992. pp. 13–29.
-
- Brener Z, Cançado J R, Galvão L M, da Luz Z M P, Filardi L D S, Pereira M E S, Santos L M T, Cançado C B. An experimental and clinical assay with ketoconazole in the treatment of Chagas disease. Mem Inst Oswaldo Cruz. 1993;88:149–153. - PubMed
-
- Brener Z, Gazzinelli R T. Immunological control of Trypanosoma cruzi infection and pathogenesis of Chagas' disease. Int Arch Allergy Immunol. 1997;114:103–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

