Bioavailability of aciclovir after oral administration of aciclovir and its prodrug valaciclovir to patients with leukopenia after chemotherapy
- PMID: 10602752
- PMCID: PMC89657
- DOI: 10.1128/AAC.44.1.207-209.2000
Bioavailability of aciclovir after oral administration of aciclovir and its prodrug valaciclovir to patients with leukopenia after chemotherapy
Abstract
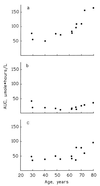
The median bioavailabilities of aciclovir after administration of aciclovir and its prodrug valaciclovir were 21.5 and 70.1%, respectively, in 12 patients with malignant hematological diseases with leukopenia after chemotherapy. The interindividual variations of the bioavailability were 48.5 and 21.0% after administration of aciclovir and valaciclovir, respectively. Neither the bioavailability nor the interindividual variation of area under the concentration-time curve of oral aciclovir or valaciclovir differed from that reported in healthy volunteers.
Figures
References
-
- Al-Yamani M, Al-Khamis J K I, El-Sayed Y M, Bawazir S A, Al-Rashood K A, Gouda M W. Comparative bioavailability of two tablet formulations of aciclovir in healthy volunteers. Int J Clin Pharmacol Ther. 1998;36:222–226. - PubMed
-
- Blum M R, Liao S H T, de Miranda P. Overview of aciclovir pharmacokinetic disposition in adults and children. Am J Med. 1982;73(Suppl. 1A):186–192. - PubMed
-
- Boxenbaum H G, Riegelman S, Elashoff R M. Statistical estimations in pharmacokinetics. J Pharm Biopharm. 1974;2:123–148. - PubMed
-
- De Miranda P, Blum M R. Pharmacokinetics of aciclovir after intravenous and oral administration. J Antimicrob Chemother. 1983;12(Suppl. B):29–37. - PubMed
-
- Dunne A. JANA: a new iterative polyexponential curve stripping program. Computer Methods Programs Biomed. 1985;20:269–275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical