Increased expression of periplasmic Cu,Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells
- PMID: 10603365
- PMCID: PMC97098
- DOI: 10.1128/IAI.68.1.30-37.2000
Increased expression of periplasmic Cu,Zn superoxide dismutase enhances survival of Escherichia coli invasive strains within nonphagocytic cells
Abstract
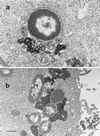
We have studied the influence of periplasmic Cu,Zn superoxide dismutase on the intracellular survival of Escherichia coli strains able to invade epithelial cells by the expression of the inv gene from Yersinia pseudotuberculosis but unable to multiply intracellularly. Intracellular viability assays, confirmed by electron microscopy observations, showed that invasive strains of E. coli engineered to increase Cu,Zn superoxide dismutase production are much more resistant to intracellular killing than strains containing only the chromosomal sodC copy. However, we have found only a slight difference in survival within HeLa cells between a sodC-null mutant and its isogenic wild-type strain. Such a small difference in survival correlates with the very low expression of this enzyme in the wild-type strain. We have also observed that acid- and oxidative stress-sensitive E. coli HB101(pRI203) is more rapidly killed in epithelial cells than E. coli GC4468(pRI203). The high mortality of E. coli HB101(pRI203), independent of the acidification of the endosome, is abolished by the overexpression of sodC. Our data suggest that oxyradicals are involved in the mechanisms of bacterial killing within epithelial cells and that high-level production of periplasmic Cu,Zn superoxide dismutase provides bacteria with an effective protection against oxidative damage. We propose that Cu,Zn superoxide dismutase could offer an important selective advantage in survival within host cells to bacteria expressing high levels of this enzyme.
Figures
References
-
- Bannister J V, Bannister W H, Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. Crit Rev Biochem. 1987;22:111–180. - PubMed
-
- Battistoni A, Rotilio G. Isolation of an active and heat-stable monomeric form of Cu,Zn superoxide dismutase from the periplasmic space of Escherichia coli. FEBS Lett. 1995;374:199–202. - PubMed
-
- Battistoni A, Donnarumma G, Greco R, Valenti P, Rotilio G. Overexpression of a hydrogen peroxide-resistant periplasmic Cu,Zn superoxide dismutase protects Escherichia coli from macrophage killing. Biochem Biophys Res Commun. 1998;243:804–807. - PubMed
-
- Battistoni A, Folcarelli S, Cervone L, Polizio F, Desideri A, Giartosio A, Rotilio G. Role of the dimeric structure in Cu,Zn superoxide dismutase. pH-dependent, reversible denaturation of the monomeric enzyme from Escherichia coli. J Biol Chem. 1998;273:5655–5661. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources