Subclinical chlamydial infection of the female mouse genital tract generates a potent protective immune response: implications for development of live attenuated chlamydial vaccine strains
- PMID: 10603387
- PMCID: PMC97120
- DOI: 10.1128/IAI.68.1.192-196.2000
Subclinical chlamydial infection of the female mouse genital tract generates a potent protective immune response: implications for development of live attenuated chlamydial vaccine strains
Abstract
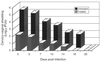
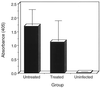
Chlamydia trachomatis is a major cause of sexually transmitted disease (STD) for which a vaccine is needed. CD4(+) T-helper type 1 (Th1) cell-mediated immunity is an important component of protective immunity against murine chlamydial genital infection. Conventional vaccine approaches have not proven effective in eliciting chlamydial-specific CD4 Th1 immunity at the genital mucosa. Thus, it is possible that the development of a highly efficacious vaccine against genital infection will depend on the generation of a live attenuated C. trachomatis vaccine. Attenuated strains of C. trachomatis do not exist, so their potential utility as vaccines cannot be tested in animal models of infection. We have developed a surrogate model to study the effect of chlamydial attenuation on infection and immunity of the female genital tract by treating mice with a subchlamydiacidal concentration of oxytetracycline following vaginal infection. Compared to untreated control mice, antibiotic-treated mice shed significantly fewer infectious organisms (3 log(10)) from the cervico-vagina, produced a minimal inflammatory response in urogenital tissue, and did not experience infection-related sequelae. Antibiotic-treated mice generated levels of chlamydia-specific antibody and cell-mediated immunity equivalent to those of control mice. Importantly, antibiotic-treated mice were found to be as immune as control untreated mice when rechallenged vaginally. These findings demonstrate that subclinical chlamydial infection of the murine female genital tract is sufficient to stimulate a potent protective immune response. They also present indirect evidence supporting the possible use of live attenuated chlamydial organisms in the development of vaccines against chlamydial STDs.
Figures



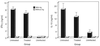
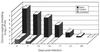
Similar articles
-
Live-attenuated influenza viruses as delivery vectors for Chlamydia vaccines.Immunology. 2007 Sep;122(1):28-37. doi: 10.1111/j.1365-2567.2007.02608.x. Epub 2007 Apr 23. Immunology. 2007. PMID: 17451464 Free PMC article.
-
The anti-idiotypic antibody to chlamydial glycolipid exoantigen (GLXA) protects mice against genital infection with a human biovar of Chlamydia trachomatis.Vaccine. 2001 Jul 16;19(28-29):4061-71. doi: 10.1016/s0264-410x(01)00117-7. Vaccine. 2001. PMID: 11427283
-
Protective efficacy of a parenterally administered MOMP-derived synthetic oligopeptide vaccine in a murine model of Chlamydia trachomatis genital tract infection: serum neutralizing IgG antibodies do not protect against chlamydial genital tract infection.Vaccine. 1995 Aug;13(11):1023-32. doi: 10.1016/0264-410x(95)00017-u. Vaccine. 1995. PMID: 8525685
-
Vaccines for Chlamydia infections of the female genital tract.Future Microbiol. 2008 Feb;3(1):67-77. doi: 10.2217/17460913.3.1.67. Future Microbiol. 2008. PMID: 18230035 Review.
-
Chlamydia trachomatis: impact on human reproduction.Hum Reprod Update. 1999 Sep-Oct;5(5):433-47. doi: 10.1093/humupd/5.5.433. Hum Reprod Update. 1999. PMID: 10582782 Review.
Cited by
-
The recall response induced by genital challenge with Chlamydia muridarum protects the oviduct from pathology but not from reinfection.Infect Immun. 2012 Jun;80(6):2194-203. doi: 10.1128/IAI.00169-12. Epub 2012 Mar 19. Infect Immun. 2012. PMID: 22431649 Free PMC article.
-
Mouse strain-dependent chemokine regulation of the genital tract T helper cell type 1 immune response.Infect Immun. 2001 Dec;69(12):7419-24. doi: 10.1128/IAI.69.12.7419-7424.2001. Infect Immun. 2001. PMID: 11705916 Free PMC article.
-
Protective immunity against mouse upper genital tract pathology correlates with high IFNγ but low IL-17 T cell and anti-secretion protein antibody responses induced by replicating chlamydial organisms in the airway.Vaccine. 2012 Jan 5;30(2):475-85. doi: 10.1016/j.vaccine.2011.10.059. Epub 2011 Nov 10. Vaccine. 2012. PMID: 22079265 Free PMC article.
-
Frequency of Chlamydia trachomatis-specific T cell interferon-γ and interleukin-17 responses in CD4-enriched peripheral blood mononuclear cells of sexually active adolescent females.J Reprod Immunol. 2014 Jun;103:29-37. doi: 10.1016/j.jri.2014.01.002. Epub 2014 Feb 1. J Reprod Immunol. 2014. PMID: 24582738 Free PMC article.
-
Immunity to murine chlamydial genital infection.Infect Immun. 2002 Jun;70(6):2741-51. doi: 10.1128/IAI.70.6.2741-2751.2002. Infect Immun. 2002. PMID: 12010958 Free PMC article. Review. No abstract available.
References
-
- Barron A L, White H J, Rank R G, Soloff B L, Moses E B. A new animal model for the study of Chlamydia trachomatis genital infections: infection of mice with the agent of mouse pneumonitis. J Infect Dis. 1981;143:63–66. - PubMed
-
- Brunham R C, Binns B, Guijon F, Danforth D, Kosseim M L, Rand F, McDowell J, Rayner E. Etiology and outcome of acute pelvic inflammatory disease. J Infect Dis. 1998;158:510–517. - PubMed
-
- Brunham R C, Peeling R, Maclean I, Kosseim M L, Paraskevas M. Chlamydia trachomatis-associated ectopic pregnancy: serologic and histologic correlates. J Infect Dis. 1992;165:1076–1081. - PubMed
-
- Chow J M, Yonekura M L, Richwald G A, Greenland S, Sweet R L, Schachter J. The association between Chlamydia trachomatis and ectopic pregnancy. JAMA. 1990;263:3164–3167. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

