In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells
- PMID: 10603407
- PMCID: PMC97140
- DOI: 10.1128/IAI.68.1.342-351.2000
In vitro Brucella suis infection prevents the programmed cell death of human monocytic cells
Abstract
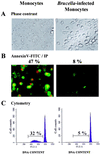
During the complex interaction between an infectious agent and a host organism, the pathogen can interfere with the host cell's programmed death to its own benefit. Induction or prevention of host cell apoptosis appears to be a critical step for determining the infection outcome. Members of the gram-negative bacterial genus Brucella are intracellular pathogens which preferentially invade monocytic cells and develop within these cells. We investigated the effect of Brucella suis infection on apoptosis of human monocytic phagocytes. The present study provides evidence that Brucella infection inhibited spontaneously occurring apoptosis in human monocytes. Prevention of monocyte apoptosis was not mediated by Brucella lipopolysaccharide and required bacterial survival within infected cells. Both invaded and noninvaded cells were protected, indicating that soluble mediators released during infection were involved in the phenomenon. Analysis of Brucella-infected monocytes revealed specific overexpression of the A1 gene, a member of the bcl-2 family implicated in the survival of hematopoietic cells. Brucella infection also rendered macrophage-like cells resistant to Fas ligand- or gamma interferon-induced apoptosis, suggesting that Brucella infection protected host cells from several cytotoxic processes occurring at different steps of the immune response. The present data clearly show that Brucella suis modulated the monocyte/macrophage's apoptotic response to the advantage of the pathogen, thus preventing host cell elimination. This might represent a strategy for Brucella development in infected hosts.
Figures



References
-
- Adachi S, Cross A R, Babior B M, Gottlieb R A. Bcl-2 and the outer mitochondrial membrane in the inactivation of cytochrome c during Fas-mediated apoptosis. J Biol Chem. 1997;272:21878–21882. - PubMed
-
- Behr-Perst S I, Munk M E, Schaberg T, Ulrichs T, Schulz R J, Kaufmann S H E. Phenotypically activated γδ T lymphocytes in the peripheral blood of patients with tuberculosis. J Infect Dis. 1999;180:141–149. - PubMed
-
- Campbell G A, Adams L G, Sowa B A. Mechanisms of binding of Brucella abortus to mononuclear phagocytes from cows naturally resistant or susceptible to brucellosis. Vet Immunol Immunopathol. 1994;41:295–306. - PubMed
-
- Caron E. Thesis. Montpellier, France: Université de Montpellier II; 1994.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

