Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart
- PMID: 10606624
- PMCID: PMC409881
- DOI: 10.1172/JCI7605
Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart
Abstract
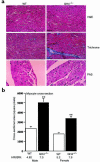
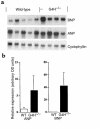
Glucose enters the heart via GLUT1 and GLUT4 glucose transporters. GLUT4-deficient mice develop striking cardiac hypertrophy and die prematurely. Whether their cardiac changes are caused primarily by GLUT4 deficiency in cardiomyocytes or by metabolic changes resulting from the absence of GLUT4 in skeletal muscle and adipose tissue is unclear. To determine the role of GLUT4 in the heart we used cre-loxP recombination to generate G4H(-/-) mice in which GLUT4 expression is abolished in the heart but is present in skeletal muscle and adipose tissue. Life span and serum concentrations of insulin, glucose, FFAs, lactate, and beta-hydroxybutyrate were normal. Basal cardiac glucose transport and GLUT1 expression were both increased approximately 3-fold in G4H(-/-) mice, but insulin-stimulated glucose uptake was abolished. G4H(-/-) mice develop modest cardiac hypertrophy associated with increased myocyte size and induction of atrial natriuretic and brain natriuretic peptide gene expression in the ventricles. Myocardial fibrosis did not occur. Basal and isoproterenol-stimulated isovolumic contractile performance was preserved. Thus, selective ablation of GLUT4 in the heart initiates a series of events that results in compensated cardiac hypertrophy.
Figures


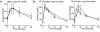


References
-
- Taegtmeyer H. Energy metabolism of the heart: from basic concepts to clinical applications. Curr Probl Cardiol. 1994;19:57–116. - PubMed
-
- Seymour AM, Eldar H, Radda GK. Hyperthyroidism results in increased glycolytic capacity in the rat heart. A 31P-NMR study. Biochim Biophys Acta. 1990;1055:107–116. - PubMed
-
- Opie LH. Myocardial ischemia—metabolic pathways and implications of increased glycolysis. Cardiovasc Drugs Ther. 1990;4:777–790. - PubMed
-
- Owen P, Dennis S, Opie LH. Glucose flux rate regulates onset of ischemic contracture in globally underperfused rat hearts. Circ Res. 1990;66:344–354. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

