Altered responsiveness to chemokines due to targeted disruption of SHIP
- PMID: 10606629
- PMCID: PMC409879
- DOI: 10.1172/JCI7310
Altered responsiveness to chemokines due to targeted disruption of SHIP
Abstract
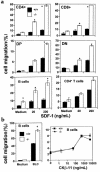
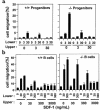
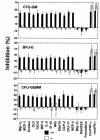
SHIP has been implicated in negative signaling in a number of hematopoietic cell types and is postulated to downregulate phosphatidylinositol-3-kinase- (PI-3K-) initiated events in diverse receptor signaling pathways. Because PI-3K is implicated in chemokine signaling, we investigated whether SHIP plays any role in cellular responses to chemokines. We found that a number of immature and mature hematopoietic cells from SHIP-deficient mice manifested enhanced directional migration (chemotaxis) in response to the chemokines stromal cell-derived factor-1 (SDF-1) and B-lymphocyte chemoattractant (BLC). SHIP(-/-) cells were also more active in calcium influx and actin polymerization in response to SDF-1. However, colony formation by SHIP-deficient hematopoietic progenitor cell (HPCs) was not inhibited by 13 myelosuppressive chemokines that normally inhibit proliferation of HPCs. These altered biologic activities of chemokines on SHIP-deficient cells are not caused by simple modulation of chemokine receptor expression in SHIP-deficient mice, implicating SHIP in the modulation of chemokine-induced signaling and downstream effects.
Figures


Similar articles
-
Heterologous regulation of chemokine receptor signaling by the lipid phosphatase SHIP in lymphocytes.Cell Signal. 2005 Oct;17(10):1194-202. doi: 10.1016/j.cellsig.2004.12.009. Cell Signal. 2005. PMID: 16038794
-
Abnormal chemokine-induced responses of immature and mature hematopoietic cells from motheaten mice implicate the protein tyrosine phosphatase SHP-1 in chemokine responses.J Exp Med. 1999 Sep 6;190(5):681-90. doi: 10.1084/jem.190.5.681. J Exp Med. 1999. PMID: 10477552 Free PMC article.
-
Stromal cell-derived factor-1/CXCL12 selectively counteracts inhibitory effects of myelosuppressive chemokines on hematopoietic progenitor cell proliferation in vitro.Stem Cells Dev. 2005 Apr;14(2):199-203. doi: 10.1089/scd.2005.14.199. Stem Cells Dev. 2005. PMID: 15910246
-
Effects of CC, CXC, C, and CX3C chemokines on proliferation of myeloid progenitor cells, and insights into SDF-1-induced chemotaxis of progenitors.Ann N Y Acad Sci. 1999 Apr 30;872:142-62; discussion 163. doi: 10.1111/j.1749-6632.1999.tb08460.x. Ann N Y Acad Sci. 1999. PMID: 10372118 Review.
-
Fc gamma RIIB activation leads to inhibition of signalling by independently ligated receptors.Biochem Soc Trans. 2003 Feb;31(Pt 1):281-5. doi: 10.1042/bst0310281. Biochem Soc Trans. 2003. PMID: 12546702 Review.
Cited by
-
Characterization of AQX-1125, a small-molecule SHIP1 activator: Part 2. Efficacy studies in allergic and pulmonary inflammation models in vivo.Br J Pharmacol. 2013 Mar;168(6):1519-29. doi: 10.1111/bph.12038. Br J Pharmacol. 2013. PMID: 23121409 Free PMC article.
-
CXCL12/CXCR4 axis in the pathogenesis of acute lymphoblastic leukemia (ALL): a possible therapeutic target.Cell Mol Life Sci. 2015 May;72(9):1715-23. doi: 10.1007/s00018-014-1830-x. Epub 2015 Jan 9. Cell Mol Life Sci. 2015. PMID: 25572297 Free PMC article. Review.
-
Subcellular localization of Grb2 by the adaptor protein Dok-3 restricts the intensity of Ca2+ signaling in B cells.EMBO J. 2007 Feb 21;26(4):1140-9. doi: 10.1038/sj.emboj.7601557. Epub 2007 Feb 8. EMBO J. 2007. PMID: 17290227 Free PMC article.
-
IL-6 increases B-cell IgG production in a feed-forward proinflammatory mechanism to skew hematopoiesis and elevate myeloid production.Blood. 2010 Jun 10;115(23):4699-706. doi: 10.1182/blood-2009-07-230631. Epub 2010 Mar 29. Blood. 2010. PMID: 20351305 Free PMC article.
-
SH2-inositol phosphatase 1 negatively influences early megakaryocyte progenitors.PLoS One. 2008;3(10):e3565. doi: 10.1371/journal.pone.0003565. Epub 2008 Oct 29. PLoS One. 2008. PMID: 18958162 Free PMC article.
References
-
- Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. - PubMed
-
- Broxmeyer HE, Kim CH. Regulation of hematopoiesis in a sea of chemokine family members with a plethora of redundant activities (review) Exp Hematol. 1999;27:1113–1123. - PubMed
-
- Kim CH, Broxmeyer HE. Chemokines: signal lamps for trafficking of T- and B-cells for development and effector function. J Leukoc Biol. 1999;65:6–15. - PubMed
-
- Strieter RM, Polverini PJ, Arenberg DA, Kunkel SL. The role of CXC chemokines as regulators of angiogenesis. Shock. 1995;4:155–160. - PubMed
-
- Murphy PM. Chemokine receptors: structure, function and role in microbial pathogenesis. Cytokine Growth Factor Rev. 1996;7:47–64. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

