RNA aptamers for the MS2 bacteriophage coat protein and the wild-type RNA operator have similar solution behaviour
- PMID: 10606647
- PMCID: PMC102504
- DOI: 10.1093/nar/28.2.489
RNA aptamers for the MS2 bacteriophage coat protein and the wild-type RNA operator have similar solution behaviour
Abstract
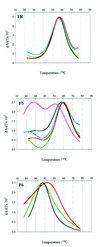
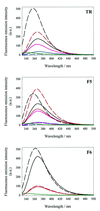
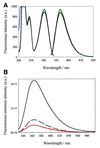
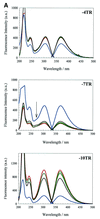
We have probed the effects of altering buffer conditions on the behaviour of two aptamer RNAs for the bacterio-phage MS2 coat protein using site-specific substitution of 2'-deoxy-2-aminopurine nucleotides at key adenosine positions. These have been compared to the wild-type operator stem-loop oligonucleotide, which is the natural target for the coat protein. The fluorescence emission spectra show a position and oligonucleotide sequence dependence which appears to reflect local conformational changes. These are largely similar between the differing oligonucleotides and deviations can be explained by the individual features of each sequence. Recognition by coat protein is enhanced, unaffected or decreased depending on the site of substitution, consistent with the known protein-RNA contacts seen in crystal structures of the complexes. These data suggest that the detailed conformational dynamics of aptamers and wild-type RNA ligands for the same protein target are remarkably similar.
Figures





References
-
- Witherall G.W., Gott,J.M. and Uhlenbeck,O.C. (1991) Prog. Nucleic Acid Res. Mol. Biol., 40, 185–220. - PubMed
-
- Borer P.N., Lin,Y., Wang,S., Roggenbuck,M.W., Gott,J.M., Uhlenbeck,O.C. and Pelczer,I. (1995) Biochemistry, 34, 6488–6503. - PubMed
-
- Nooren I.M.A., Wang,K.Y., Borer,P.N. and Pelczer,I. (1998) J. Biomol. NMR, 11, 319–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

