Expression of costimulatory molecules CD80 and CD86 and their receptors CD28, CTLA-4 on malignant ascites CD3+ tumour-infiltrating lymphocytes (TIL) from patients with ovarian and other types of peritoneal carcinomatosis
- PMID: 10606960
- PMCID: PMC1905534
- DOI: 10.1046/j.1365-2249.2000.01105.x
Expression of costimulatory molecules CD80 and CD86 and their receptors CD28, CTLA-4 on malignant ascites CD3+ tumour-infiltrating lymphocytes (TIL) from patients with ovarian and other types of peritoneal carcinomatosis
Abstract
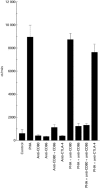
Costimulation of T lymphocytes by the leucocyte surface molecules CD80 and CD86 expressed on antigen-presenting cells (APC) is required for the development of T cell responses. The CD28 and CTLA-4 molecules on T cells serve as receptors for the CD80 and CD86 costimulatory antigens. We have examined the frequency of expression of CD80 (B7.1), CD86 (B7.2), CD28 and CTLA-4 surface antigens on TIL isolated from malignant ascites or peritoneal washings of 26 patients with ovarian carcinoma and five patients with non-ovarian peritoneal carcinomatosis. Expression of CD80 and CD86 antigen was detected by reverse transcription-polymerase chain reaction (RT-PCR), and by FACS analysis. Significantly higher proportions of intraperitoneal CD3+ cells expressed CD86 antigen than the CD80 antigen (14 +/- 9% versus 3 +/- 3%, P < 0.05). Moreover, CD3+CD86+ cells were significantly more frequent in the peritoneal fluid (14 +/- 9%) than in the peripheral blood (3 +/- 0.4%, P < 0.05) of ovarian patients or normal controls (3 +/- 1%). CTLA-4 and CD28 antigen were expressed, respectively, on 9 +/- 4% and 86 +/- 14% of ascitic CD3+ cells of ovarian cancer patients. Both CD80 and CD86 antigens were expressed primarily on HLA-DR+ ascites TIL and were present in a very low proportion of HLA-DR- ascites TIL. These HLA-DR+ cells may represent a population of lymphocytes that have been activated in vivo, and function as APC. An anti-CD86 MoAb or a combination of anti-CD86 and anti-CD80 MoAbs significantly inhibited the proliferation of cultured intraperitoneal TIL. We have shown that in addition to CD28 and CTLA-4, CD3+ intraperitoneal TIL express the costimulatory molecules CD80 and CD86. The expression of these molecules on T cells could be dependent upon certain factors in the tumour microenvironment that could determine the outcome of in vivo immune responses.
Figures

Similar articles
-
Activation of human peripheral blood dendritic cells induces the CD86 co-stimulatory molecule.Eur J Immunol. 1995 Jul;25(7):2064-8. doi: 10.1002/eji.1830250739. Eur J Immunol. 1995. PMID: 7542604
-
Covalent dimerization of CD28/CTLA-4 and oligomerization of CD80/CD86 regulate T cell costimulatory interactions.J Biol Chem. 1996 Oct 25;271(43):26762-71. doi: 10.1074/jbc.271.43.26762. J Biol Chem. 1996. PMID: 8900156
-
Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors.Immunity. 1994 Dec;1(9):793-801. doi: 10.1016/s1074-7613(94)80021-9. Immunity. 1994. PMID: 7534620
-
Regulation of T and B cell responses by modulating interactions between CD28/CTLA4 and their ligands, CD80 and CD86.Ann N Y Acad Sci. 1997 Apr 5;815:392-400. doi: 10.1111/j.1749-6632.1997.tb52090.x. Ann N Y Acad Sci. 1997. PMID: 9186685 Review.
-
CD80 (B7-1) and CD86 (B7-2): potential targets for immunotherapy?Res Immunol. 1995 Mar-Apr;146(3):183-96. doi: 10.1016/0923-2494(96)80256-2. Res Immunol. 1995. PMID: 8525052 Review. No abstract available.
Cited by
-
Boosting the Immune Response-Combining Local and Immune Therapy for Prostate Cancer Treatment.Cells. 2022 Sep 7;11(18):2793. doi: 10.3390/cells11182793. Cells. 2022. PMID: 36139368 Free PMC article. Review.
-
Trends in peritoneal surface malignancies: evidence from a Czech nationwide population-based study.World J Surg Oncol. 2019 Nov 6;17(1):182. doi: 10.1186/s12957-019-1731-4. World J Surg Oncol. 2019. PMID: 31694646 Free PMC article.
-
Dysregulated expression of both the costimulatory CD28 and inhibitory CTLA-4 molecules in PB T cells of advanced cervical cancer patients suggests systemic immunosuppression related to disease progression.Pathol Oncol Res. 2012 Apr;18(2):479-89. doi: 10.1007/s12253-011-9471-y. Epub 2011 Nov 19. Pathol Oncol Res. 2012. PMID: 22094905 Free PMC article.
-
Vitamin D Controls Tumor Growth and CD8+ T Cell Infiltration in Breast Cancer.Front Immunol. 2019 Jun 6;10:1307. doi: 10.3389/fimmu.2019.01307. eCollection 2019. Front Immunol. 2019. PMID: 31244851 Free PMC article.
-
Interaction Landscape of Inherited Polymorphisms with Somatic Events in Cancer.Cancer Discov. 2017 Apr;7(4):410-423. doi: 10.1158/2159-8290.CD-16-1045. Epub 2017 Feb 10. Cancer Discov. 2017. PMID: 28188128 Free PMC article.
References
-
- Marrack P, Kappler J. The T cell receptor. Science. 1987;238:1073–9. - PubMed
-
- Geppert TD, Davis LS, Gur H, Wacholtz MD, Lipsky PE. Accessory cell signals involved in T-cell activation. Immunol Rev. 1990;117:5–66. - PubMed
-
- Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen. Annu Rev Immunol. 1993;11:191–212. - PubMed
-
- June CH, Bluestone JA, Nadler LM, Thompson CB. The B7 and CD28 receptor families. Immunol Today. 1994;15:321–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

