Induction of microthrombotic thrombocytopenia in normal mice by transferring a platelet-reactive, monoclonal anti-gp70 autoantibody established from MRL/lpr mice: an autoimmune model of thrombotic thrombocytopenic purpura
- PMID: 10606963
- PMCID: PMC1905520
- DOI: 10.1046/j.1365-2249.2000.01116.x
Induction of microthrombotic thrombocytopenia in normal mice by transferring a platelet-reactive, monoclonal anti-gp70 autoantibody established from MRL/lpr mice: an autoimmune model of thrombotic thrombocytopenic purpura
Abstract
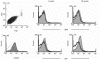
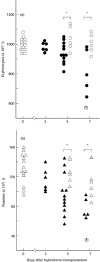
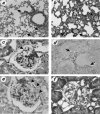
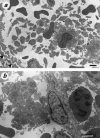
MRL/MpJ-lpr/lpr (MRL/lpr) mice spontaneously develop immune complex-mediated glomerulonephritis and thrombocytopenia. Although the presence of cross-reactive anti-phospholipid antibodies in sera of MRL/lpr mice has been demonstrated, possible relationships between detected autoantibodies and the development of thrombocytopenia have not been elucidated. Recent genetic analyses in a few different strains of lupus-prone mice have pointed out a close correlation between autoantibodies reactive with endogenous retroviral env gene product, gp70, and the development and severity of glomerulonephritis. In the process of establishing possibly nephritogenic anti-gp70 autoantibody-producing hybridoma cells from MRL/lpr mice, we identified an IgG2a-producing anti-gp70 hybridoma clone that induced microvascular intraluminal platelet aggregation, thrombocytopenia, and amenia upon transplantation into syngeneic non-autoimmune mice. This and two other anti-gp70 antibodies bound onto the surface of mouse platelets, and purified IgG2a of the anti-gp70 autoantibody induced glomerular lesions with characteristics of thrombotic thrombocytopenic purpura when injected into non-autoimmune mice. The pathogenic anti-gp70 autoantibody specifically precipitated a platelet protein with an approximate relative molecular mass of 40 000.
Figures

References
-
- Moake JL. Studies on the pathophysiology of thrombotic thrombocytopenic purpura. Semin Hematol. 1997;34:83–9. - PubMed
-
- Ruggenenti P, Luts J, Remuzzi G. Pathogenesis and treatment of thrombotic microangiopathy. Kidney Int. 1997;51(Suppl. 58):S97–S101. - PubMed
-
- Neild GH. Hemolytic uremic syndrome/thrombotic thrombocytopenic purpura. pathophysiology and treatment. Kidney Int. 1998;53(Suppl. 64):S45–S49. - PubMed
-
- Tandon NN, Rock G, Jamieson GA. Anti-CD36 antibodies in thrombotic thrombocytopenia purpura. Br J Haematol. 1994;88:816–25. - PubMed
-
- Schultz DR, Arnold PI, Jy W, et al. Anti-CD36 autoantibodies in thrombotic thrombocytopenic purpura and other thrombotic disorders: identification of an 85kD form of CD36 as a target antigen. Br J Haematol. 1998;103:849–57. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

