Molecular determinants of polyreactive antibody binding: HCDR3 and cyclic peptides
- PMID: 10606966
- PMCID: PMC1905533
- DOI: 10.1046/j.1365-2249.2000.01096.x
Molecular determinants of polyreactive antibody binding: HCDR3 and cyclic peptides
Abstract
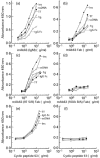
Human monoclonal antibody 63 (mAb63) is an IgM/lambda polyreactive antibody that binds to multiple self and non-self antigens. The molecular basis of polyreactivity is still unclear. The present study was initiated to prepare a recombinant Fab of mAb63 and use it to study the determinants involved in polyreactivity. The baculovirus system was employed to express large amounts of mAb63 Fab in Sf9 cells. Our experiments showed that infected Sf9 cells secreted a soluble 50-kD Fab heterodimer that bound to multiple self and non-self antigens. The antigen-binding activity of mAb63 Fab was inhibited by both homologous and heterologous antigens. To study in more detail the molecular determinants involved in polyreactivity, the heavy chain complementarity-determining region 3 (HCDR3), which is known to play a key role in the binding of monoreactive antibodies to antigens, was subjected to site-directed mutagenesis. A single substitution, alanine for arginine, at position 100A resulted in complete loss of antigen-binding activity. The 19 amino acids comprising the HCDR3 of mAb63 were then synthesized and a cyclic peptide prepared. The cyclic peptide showed the same antigen-binding pattern as the parental mAb63 and the recombinant mAb63 Fab. A five amino acid motif (RFLEW), present in the HCDR3 of mAb63, was found by searching the GenBank in three of 50 other human polyreactive antibodies, but in none of nearly 2500 human antibodies thought to be monoreactive. It is concluded that HCDR3 plays a major role in polyreactivity and that in some cases cyclic peptides comprising the HCDR3, by themselves, may be polyreactive.
Figures

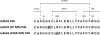

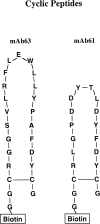
Similar articles
-
Determinants of polyreactivity in a large panel of recombinant human antibodies from HIV-1 infection.J Immunol. 1996 Jul 15;157(2):739-49. J Immunol. 1996. PMID: 8752924
-
Both VH and VL chains of polyreactive IgM antibody are required for polyreactivity: expression of Fab in Escherichia coli.Clin Exp Immunol. 1995 Aug;101(2):383-6. doi: 10.1111/j.1365-2249.1995.tb08368.x. Clin Exp Immunol. 1995. PMID: 7648724 Free PMC article.
-
Analysis of IgE antibodies from a patient with atopic dermatitis: biased V gene usage and evidence for polyreactive IgE heavy chain complementarity-determining region 3.J Immunol. 2002 Jun 15;168(12):6305-13. doi: 10.4049/jimmunol.168.12.6305. J Immunol. 2002. PMID: 12055246
-
Regulation of the antibody repertoire through control of HCDR3 diversity.Vaccine. 1998 Aug-Sep;16(14-15):1383-90. doi: 10.1016/s0264-410x(98)00096-6. Vaccine. 1998. PMID: 9711776 Free PMC article. Review.
-
Comparison of the three-dimensional structures of a humanized and a chimeric Fab of an anti-gamma-interferon antibody.J Mol Recognit. 1999 Jan-Feb;12(1):19-32. doi: 10.1002/(SICI)1099-1352(199901/02)12:1<19::AID-JMR445>3.0.CO;2-Y. J Mol Recognit. 1999. PMID: 10398393 Review.
Cited by
-
Uncovering temporospatial sensitive TBI targeting strategies via in vivo phage display.Sci Adv. 2022 Jul 22;8(29):eabo5047. doi: 10.1126/sciadv.abo5047. Epub 2022 Jul 22. Sci Adv. 2022. PMID: 35867794 Free PMC article.
-
B-Cell Memory Responses to Variant Viral Antigens.Viruses. 2021 Mar 26;13(4):565. doi: 10.3390/v13040565. Viruses. 2021. PMID: 33810456 Free PMC article. Review.
-
The natural antibody repertoire of sharks and humans recognizes the potential universe of antigens.Protein J. 2004 Feb;23(2):103-18. doi: 10.1023/b:jopc.0000020077.73751.76. Protein J. 2004. PMID: 15106876
-
Immunoglobulin diversity gene usage predicts unfavorable outcome in a subset of chronic lymphocytic leukemia patients.J Clin Invest. 2008 Jan;118(1):306-15. doi: 10.1172/JCI32625. J Clin Invest. 2008. PMID: 18064298 Free PMC article.
-
Structure-based approaches to inhibition of erbB receptors with peptide mimetics.Immunol Res. 2003;27(2-3):303-8. doi: 10.1385/IR:27:2-3:303. Immunol Res. 2003. PMID: 12857977 Review.
References
-
- Haspel MV, Onodera T, Prabhakar BS, McClintock PR, Essani K, Ray UR, Yagihashi S, Notkins AL. Multiple organ-reactive monoclonal autoantibodies. Nature. 1983;304:73–6. - PubMed
-
- Satoh J, Prabhakar BS, Haspel MV, Ginsberg-Fellner F, Notkins AL. Human monoclonal autoantibodies that react with multiple endocrine organs. N Engl J Med. 1983;309:217–20. - PubMed
-
- Dighiero G, Lymberi P, Mazie JC, Rouyre S, Butler-Browne GS, Whalen RG, Avrameas S. Murine hybridomas secreting natural monoclonal antibodies reacting with self antigens. J Immunol. 1983;131:2267–72. - PubMed
-
- Casali P, Notkins AL. Probing the human B-cell repertoire with EBV: polyreactive antibodies and CD5+ B lymphocytes. Ann Rev Immunol. 1989;7:513–35. - PubMed
-
- Casali P, Burastero SS, Balow JE, Notkins AL. High-affinity antibodies to ssDNA are produced by CD− B cells in systemic lupus erythematosus patients. J Immunol. 1989;143:3476–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

