Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA)
- PMID: 10606983
- PMCID: PMC1905539
- DOI: 10.1046/j.1365-2249.2000.01110.x
Expression and production of the long pentraxin PTX3 in rheumatoid arthritis (RA)
Abstract
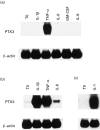
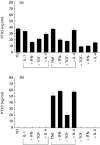
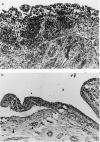
PTX3 is a secreted molecule which consists of a C-terminal domain similar to classical pentraxins (e.g. C-reactive protein (CRP)) and of an unrelated N-terminal domain. Unlike the classical pentraxins, the long pentraxin PTX3 is expressed in response to IL-1beta and tumour necrosis factor-alpha (TNF-alpha), but not to IL-6, in various cell types. The present study was designed to investigate the expression of PTX3 in RA. Dissociated RA and osteoarthritis (OA) type B synoviocytes were cultured in the presence and in the absence of inflammatory cytokines. PTX3 mRNA expression in synoviocytes was evaluated by Northern analysis. PTX3 protein levels in synovial cell cultures and synovial fluid were estimated by ELISA, and PTX3 distribution in synovial tissues by immunohistochemical techniques. OA synoviocytes were induced to express high levels of PTX3 mRNA by TNF-alpha, but not by other cytokines including IL-1beta and IL-6. RA synoviocytes, unlike OA synoviocytes, constitutively expressed high levels of PTX3 in the absence of deliberate stimulation. The constitutive expression of PTX3 in RA synoviocytes was not modified by anti-TNF-alpha antibodies, IL-1 receptor antagonist or a combination of the two agents. In contrast, interferon-gamma and transforming growth factor-beta inhibited PTX3 constitutive expression in RA synoviocytes. The joint fluid from RA patients contained higher levels of immunoreactive PTX3 than controls and the synovial tissue contained endothelial cells and synoviocytes positive for PTX3 by immunohistochemistry. In conclusion, PTX3 may play a role in inflammatory circuits of RA, and its relevance as a marker of disease activity deserves further study.
Figures



Similar articles
-
Perspectives on long pentraxin 3 and rheumatoid arthritis: several potential breakthrough points relying on study foundation of the past.Int J Med Sci. 2021 Mar 3;18(8):1886-1898. doi: 10.7150/ijms.54787. eCollection 2021. Int J Med Sci. 2021. PMID: 33746606 Free PMC article. Review.
-
Serum amyloid A (SAA) induces pentraxin 3 (PTX3) production in rheumatoid synoviocytes.Mod Rheumatol. 2013 Jan;23(1):28-35. doi: 10.1007/s10165-012-0630-0. Epub 2012 Mar 24. Mod Rheumatol. 2013. PMID: 22447522
-
Scleroderma fibroblasts constitutively express the long pentraxin PTX3.Clin Exp Rheumatol. 2004 Jan-Feb;22(3 Suppl 33):S66-72. Clin Exp Rheumatol. 2004. PMID: 15344601
-
Tumor necrosis factor-alpha induces vascular endothelial growth factor-C expression in rheumatoid synoviocytes.J Rheumatol. 2007 Jan;34(1):16-9. J Rheumatol. 2007. PMID: 17216674
-
Arthritic and non-arthritic synovial fluids modulate IL10 and IL1RA gene expression in differentially activated primary human monocytes.Osteoarthritis Cartilage. 2015 Nov;23(11):1853-7. doi: 10.1016/j.joca.2015.06.003. Osteoarthritis Cartilage. 2015. PMID: 26521731 Review.
Cited by
-
Association of Genetic Variants in Pentraxin 3 Gene with Ankylosing Spondylitis.Med Sci Monit. 2016 Aug 18;22:2911-6. doi: 10.12659/msm.896562. Med Sci Monit. 2016. PMID: 27538101 Free PMC article.
-
Perspectives on long pentraxin 3 and rheumatoid arthritis: several potential breakthrough points relying on study foundation of the past.Int J Med Sci. 2021 Mar 3;18(8):1886-1898. doi: 10.7150/ijms.54787. eCollection 2021. Int J Med Sci. 2021. PMID: 33746606 Free PMC article. Review.
-
Increased mortality and inflammation in tumor necrosis factor-stimulated gene-14 transgenic mice after ischemia and reperfusion injury.Am J Pathol. 2002 May;160(5):1755-65. doi: 10.1016/s0002-9440(10)61122-4. Am J Pathol. 2002. PMID: 12000727 Free PMC article.
-
The yin-yang of long pentraxin PTX3 in inflammation and immunity.Immunol Lett. 2014 Sep;161(1):38-43. doi: 10.1016/j.imlet.2014.04.012. Epub 2014 May 1. Immunol Lett. 2014. PMID: 24792672 Free PMC article. Review.
-
Investigating the molecular control of deer antler extract on articular cartilage.J Orthop Surg Res. 2021 Jan 6;16(1):8. doi: 10.1186/s13018-020-02148-w. J Orthop Surg Res. 2021. PMID: 33407721 Free PMC article.
References
-
- Harris ED. Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990;322:1277–89. - PubMed
-
- Fassbender HG. Histomorphologic basis of articular cartilage destruction in rheumatoid arthritis. Coll Relat Res. 1983;3:141–51. - PubMed
-
- Firestein GS, Zvaifler NJ. Rheumatoid arthritis: a disease of disordered immunity. In: Gallin JI, Goldstein IM, Willis K, editors. Inflammation: basic principles and clinical correlates. 2. New York: Raven Press; 1992.
-
- Rammers EF, Sano H, Wilder R. Platelet-derived growth factors and heparin-binding (fibroblasts) growth factors in the synovial pathology of rheumatoid arthritis. Sem Arthritis Rheum. 1991;21:191–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

