Vascular endothelial growth factor overproduced by tumour cells acts predominantly as a potent angiogenic factor contributing to malignant progression
- PMID: 10607018
- PMCID: PMC2517834
- DOI: 10.1046/j.1365-2613.1999.00122.x
Vascular endothelial growth factor overproduced by tumour cells acts predominantly as a potent angiogenic factor contributing to malignant progression
Abstract
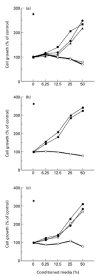
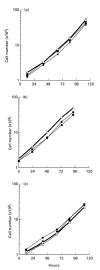
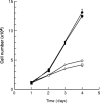
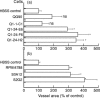
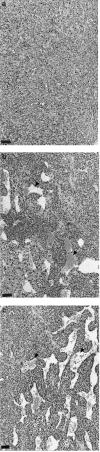
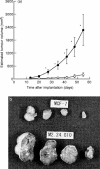
To elucidate the role of vascular endothelial growth factor (VEGF), an endothelial cell-specific mitogen, in tumour angiogenesis and malignant progression, an expression vector harboring human VEGF cDNA was stably transfected into three human cancer cell lines with poor VEGF productivity. Though their in vitro growth rate and intrinsic productivity of another angiogenic factor, basic fibroblast growth factor (bFGF), were not changed by transfection, those clones with higher VEGF production were endowed with tumorigenic and angiogenic potentials as follows: firstly, nontumorigenic, lung carcinoma QG90 cells having lower bFGF productivity acquired tumorigenicity as well as significant in vivo angiogenesis-inducing ability, secondly, tumorigenic colorectal carcinoma RPMI4788 cells having higher potency for bFGF production could form more vascularized solid tumour with faster growth rate and thirdly, oestrogen-dependent breast carcinoma MCF-7 cells, which did not produce detectable bFGF, acquired tumorigenicity even in the absence of oestrogen and the solid tumour growth rate was remarkably enhanced, accompanied with increased vascularization, in the presence of oestrogen. These results suggest that tumour progression closely depends on angiogenesis, and VEGF significantly contributes to malignant progression of a variety of tumour cells through its potent angiogenic activity, independent on the bFGF productivity of tumour cells.
Figures


Similar articles
-
Expression of vascular permeability factor/vascular endothelial growth factor by melanoma cells increases tumor growth, angiogenesis, and experimental metastasis.Cancer Res. 1996 Jan 1;56(1):172-81. Cancer Res. 1996. PMID: 8548760
-
Modulation of angiogenesis and tumorigenicity of human melanocytic cells by vascular endothelial growth factor and basic fibroblast growth factor.Cancer Res. 2001 Oct 1;61(19):7282-90. Cancer Res. 2001. PMID: 11585767
-
Enhancement of tumor growth and vascular density by transfection of vascular endothelial cell growth factor into MCF-7 human breast carcinoma cells.J Natl Cancer Inst. 1995 Feb 1;87(3):213-9. doi: 10.1093/jnci/87.3.213. J Natl Cancer Inst. 1995. PMID: 7535859
-
Transfected MCF-7 cells as a model for breast-cancer progression.Breast Cancer Res Treat. 1994;31(2-3):153-65. doi: 10.1007/BF00666149. Breast Cancer Res Treat. 1994. PMID: 7881095 Review.
-
Targetting VEGF in anti-angiogenic and anti-tumour therapy: where are we now?Int J Exp Pathol. 1998 Dec;79(6):339-46. doi: 10.1046/j.1365-2613.1998.00086.x. Int J Exp Pathol. 1998. PMID: 10319015 Free PMC article. Review.
Cited by
-
RLIP76 regulates HIF-1 activity, VEGF expression and secretion in tumor cells, and secretome transactivation of endothelial cells.FASEB J. 2014 Sep;28(9):4158-68. doi: 10.1096/fj.14-255711. Epub 2014 Jun 13. FASEB J. 2014. PMID: 24928198 Free PMC article.
-
The MSC-MCF-7 Duet Playing Tumor Vasculogenesis and Angiogenesis onto the Chick Embryo Chorioallantoic Membrane.In Vivo. 2020 Nov-Dec;34(6):3315-3325. doi: 10.21873/invivo.12170. In Vivo. 2020. PMID: 33144439 Free PMC article.
-
Pathophysiology of ocular surface squamous neoplasia.Exp Eye Res. 2014 Dec;129:172-82. doi: 10.1016/j.exer.2014.10.015. Epub 2014 Oct 18. Exp Eye Res. 2014. PMID: 25447808 Free PMC article. Review.
-
Differential Angiogenic Responses of Human Endothelial Colony-Forming Cells to Different Molecular Subtypes of Breast Cancer Cells.J Lipid Atheroscler. 2021 Jan;10(1):111-122. doi: 10.12997/jla.2021.10.1.111. Epub 2021 Jan 18. J Lipid Atheroscler. 2021. PMID: 33537258 Free PMC article.
-
In Vitro Vascular Network Modified to Function as Culture Platform and Angiogenic Induction Potential Test for Cancer Cells.Int J Mol Sci. 2020 Mar 6;21(5):1833. doi: 10.3390/ijms21051833. Int J Mol Sci. 2020. PMID: 32155897 Free PMC article.
References
-
- Abraham JA, Mergia A, Whang JL, et al. Nucleotide sequence of a bovine clone encoding the angiogenic protein, basic fibroblast growth factor. Science. 1986;233:545–548. - PubMed
-
- Aonuma M, Iwahana M, Nakayama Y, et al. Tumorigenicity depends on angiogenic potential of tumor cells: dominant role of vascular endothelial growth factor and/or fibroblast growth factors produced by tumor cells. Angiogenesis. 1998;2:57–66. - PubMed
-
- Bosari S, Lee AK, Delellis RA, Wiley BD, Heatley GJ, Silverman ML. Microvessel quantitation and prognosis in invasive breast carcinoma. Hum. Pathol. 1992;23:755–761. - PubMed
-
- Brown L, Berse B, Jackman R, Schnitt S, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum. Pathol. 1995;26:86–91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

