HIV-1 genotypic resistance patterns predict response to saquinavir-ritonavir therapy in patients in whom previous protease inhibitor therapy had failed
- PMID: 10610625
- PMCID: PMC2606144
- DOI: 10.7326/0003-4819-131-11-199912070-00003
HIV-1 genotypic resistance patterns predict response to saquinavir-ritonavir therapy in patients in whom previous protease inhibitor therapy had failed
Abstract
Background: Tests for resistance to HIV drugs are available for clinical use; however, their predictive value has not been fully assessed.
Objectives: To determine HIV-1 genotypic predictors of a virologic response to saquinavir-ritonavir therapy in patients in whom at least one previous protease inhibitor-containing regimen had failed and to compare the predictive value of baseline genotype with that of standard clinical evaluation.
Design: Retrospective clinical cohort study.
Setting: University-based HIV clinic.
Patients: 54 HIV-1-infected adults treated with saquinavir-ritonavir who had experienced virologic failure while receiving a protease inhibitor-containing regimen for at least 3 months.
Measurements: HIV-1 reverse transcriptase and protease gene sequences, CD4 cell counts, clinical characteristics, detailed antiretroviral treatment history, and plasma HIV-1 RNA levels at baseline and at three follow-up time points (median, 4, 12, and 26 weeks). Virologic failure was defined as a plasma HIV RNA level greater than 1000 copies/mL.
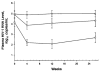
Results: In 22 patients (41%), a plasma HIV-1 RNA level less than 500 copies/mL was achieved by week 12; in 15 patients (28%), this response was maintained through week 26. Clinical characteristics predicting a poorer response included a diagnosis of AIDS, lower CD4 cell count, and higher plasma HIV RNA level (P<0.03). Number of previous nucleoside reverse transcriptase inhibitors, previous protease inhibitor therapy, and duration of previous protease inhibitor therapy were predictors of poorer response (P<0.01). Multivariate regression models revealed that protease mutations present at the initiation of saquinavir-ritonavir therapy were the strongest predictors of virologic response. A model of clinical features explained up to 45% of the variation in virologic outcomes by week 12, whereas the explained variance was 71% when genotypic predictors were included.
Conclusions: In patients in whom protease inhibitor-containing antiretroviral therapy fails, HIV-1 genotype is predictive of virologic response to subsequent therapy. This predictive capacity adds to that of standard clinical evaluation.
Figures

References
-
- Gulick RM, Mellors JW, Havlir D, Eron JJ, Gonzalez C, McMahon D, et al. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N Engl J Med. 1997;337:734–9. - PubMed
-
- Hammer SM, Squires KE, Hughes MD, Grimes JM, Demeter LM, Currier JS, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med. 1997;337:725–33. - PubMed
-
- Montaner JS, Reiss P, Cooper D, Vella S, Harris M, Conway B, et al. A randomized, double-blind trial comparing combinations of nevirapine, didanosine, and zidovudine for HIV-infected patients: the INCAS trial. The Netherlands, Canada and Australia Study. JAMA. 1998;279:930–7. - PubMed
-
- Cameron DW, Heath-Chiozzi M, Danner S, Cohen C, Kravcik S, Maurath C, et al. Randomized placebo-controlled trial of ritonavir in advanced HIV-1 disease. The Advanced HIV Disease Ritonavir Study Group. Lancet. 1998;351:543–9. - PubMed
-
- Powderly WG, Landay A, Lederman MM. Recovery of the immune system with antiretroviral therapy: the end of opportunism? JAMA. 1998;280:72–7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
