The transmembrane mutation G380R in fibroblast growth factor receptor 3 uncouples ligand-mediated receptor activation from down-regulation
- PMID: 10611230
- PMCID: PMC85119
- DOI: 10.1128/MCB.20.2.516-522.2000
The transmembrane mutation G380R in fibroblast growth factor receptor 3 uncouples ligand-mediated receptor activation from down-regulation
Abstract
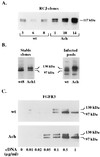
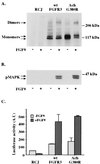
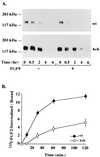
A point mutation, Gly380Arg, in the transmembrane domain of fibroblast growth factor receptor 3 (FGFR3) leads to achondroplasia, the most common form of genetic dwarfism in humans. This substitution was suggested to enhance mutant receptor dimerization, leading to constitutive, ligand-independent activation. We found that dimerization and activation of the G380R mutant receptor are predominantly ligand dependent. However, using both transient and stable transfections, we found significant overexpression only of the mutant receptor protein. Metabolic pulse-chase experiments, cell surface labeling, and kinetics of uptake of radiolabeled ligand demonstrated a selective delay in the down-regulation of the mutant receptor. Moreover, this receptor was now resistant to ligand-mediated internalization, even at saturating ligand concentrations. Finally, transgenic mice expressing the human G380R mutant receptor under the mouse receptor transcriptional control demonstrated a markedly expanded area of FGFR3 immunoreactivity within their epiphyseal growth plates, compatible with an in vivo defect in receptor down-regulation. We propose that the achondroplasia mutation G380R uncouples ligand-mediated receptor activation from down-regulation at a site where the levels and kinetics of FGFR3 signals are crucial for chondrocyte maturation and bone formation.
Figures



References
-
- Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. - PubMed
-
- Bellus G A, McIntosh I, Szabo J, Aylsworth A, Kaitila I, Francomano C A. Hypochondroplasia: molecular analysis of the fibroblast growth factor receptor 3 gene. Ann N Y Acad Sci. 1996;785:182–187. - PubMed
-
- Cancedda R, Descalzi Cancedda F, Castagnola P. Chondrocyte differentiation. Int Rev Cytol. 1995;159:265–358. - PubMed
-
- Colvin J S, Bohne B A, Harding G W, McEwen D G, Ornitz D M. Skeletal overgrowth and deafness in mice lacking fibroblast growth factor receptor 3. Nat Genet. 1996;12:390–397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
