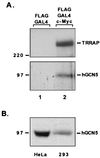
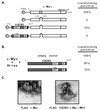
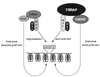
The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc
- PMID: 10611234
- PMCID: PMC85131
- DOI: 10.1128/MCB.20.2.556-562.2000
The essential cofactor TRRAP recruits the histone acetyltransferase hGCN5 to c-Myc
Abstract
The c-Myc protein functions as a transcription factor to facilitate oncogenic transformation; however, the biochemical and genetic pathways leading to transformation remain undefined. We demonstrate here that the recently described c-Myc cofactor TRRAP recruits histone acetylase activity, which is catalyzed by the human GCN5 protein. Since c-Myc function is inhibited by recruitment of histone deacetylase activity through Mad family proteins, these opposing biochemical activities are likely to be responsible for the antagonistic biological effects of c-Myc and Mad on target genes and ultimately on cellular transformation.
Figures




References
-
- Ayer D E, Kretzner L, Eisenman R N. Mad: a heterodimeric partner for max that antagonizes myc transcriptional activity. Cell. 1993;72:211–222. - PubMed
-
- Ayer D E, Lawrence Q A, Eisenman R N. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell. 1995;80:767–776. - PubMed
-
- Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. - PubMed
-
- Bouchard C, Staller P, Eilers M. Control of cell proliferation by Myc. Trends Cell Biol. 1998;8:202–206. - PubMed
-
- Brehm A, Miska E A, McCance D J, Reid J L, Bannister A J, Kouzarides T. Retinoblastoma protein recruits histone deacetylase to repress transcription. Nature. 1998;391:597–601. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
