The heme-copper oxidases of Thermus thermophilus catalyze the reduction of nitric oxide: evolutionary implications
- PMID: 10611279
- PMCID: PMC24714
- DOI: 10.1073/pnas.96.26.14718
The heme-copper oxidases of Thermus thermophilus catalyze the reduction of nitric oxide: evolutionary implications
Abstract
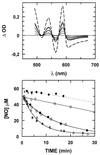
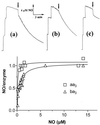
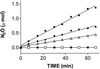
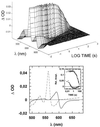
We show that the heme-copper terminal oxidases of Thermus thermophilus (called ba(3) and caa(3)) are able to catalyze the reduction of nitric oxide (NO) to nitrous oxide (N(2)O) under reducing anaerobic conditions. The rate of NO consumption and N(2)O production were found to be linearly dependent on enzyme concentration, and activity was abolished by enzyme denaturation. Thus, contrary to the eukaryotic enzyme, both T. thermophilus oxidases display a NO reductase activity (3.0 +/- 0.7 mol NO/mol ba(3) x min and 32 +/- 8 mol NO/mol caa(3) x min at [NO] approximately 50 microM and 20 degrees C) that, though considerably lower than that of bona fide NO reductases (300-4,500 mol NO/mol enzyme x min), is definitely significant. We also show that for ba(3) oxidase, NO reduction is associated to oxidation of cytochrome b at a rate compatible with turnover, suggesting a mechanism consistent with the stoichiometry of the overall reaction. We propose that the NO reductase activity of T. thermophilus oxidases may depend on a peculiar Cu(B)(+) coordination, which may be revealed by the forthcoming three-dimensional structure. These findings support the hypothesis of a common phylogeny of aerobic respiration and bacterial denitrification, which was proposed on the basis of structural similarities between the Pseudomonas stutzeri NO reductase and the cbb(3) terminal oxidases. Our findings represent functional evidence in support of this hypothesis.
Figures
Similar articles
-
Fourier transform infrared characterization of a CuB-nitrosyl complex in cytochrome ba3 from Thermus thermophilus: relevance to NO reductase activity in heme-copper terminal oxidases.J Am Chem Soc. 2007 Dec 5;129(48):14952-8. doi: 10.1021/ja074600a. Epub 2007 Nov 13. J Am Chem Soc. 2007. PMID: 17997553 Free PMC article.
-
NO binding and dynamics in reduced heme-copper oxidases aa3 from Paracoccus denitrificans and ba3 from Thermus thermophilus.Biochemistry. 2004 Nov 9;43(44):14118-27. doi: 10.1021/bi0488808. Biochemistry. 2004. PMID: 15518562
-
The cytochrome cbb3 from Pseudomonas stutzeri displays nitric oxide reductase activity.Eur J Biochem. 2001 Dec;268(24):6486-91. doi: 10.1046/j.0014-2956.2001.02597.x. Eur J Biochem. 2001. PMID: 11737203
-
Proton transfer in ba(3) cytochrome c oxidase from Thermus thermophilus.Biochim Biophys Acta. 2012 Apr;1817(4):650-7. doi: 10.1016/j.bbabio.2011.11.015. Epub 2011 Dec 7. Biochim Biophys Acta. 2012. PMID: 22172736 Review.
-
Bioenergetics at extreme temperature: Thermus thermophilus ba(3)- and caa(3)-type cytochrome c oxidases.Biochim Biophys Acta. 2012 Apr;1817(4):638-49. doi: 10.1016/j.bbabio.2011.08.004. Biochim Biophys Acta. 2012. PMID: 22385645 Review.
Cited by
-
Substrate control of internal electron transfer in bacterial nitric-oxide reductase.J Biol Chem. 2010 Aug 13;285(33):25531-7. doi: 10.1074/jbc.M110.123984. Epub 2010 Jun 11. J Biol Chem. 2010. PMID: 20547487 Free PMC article.
-
The Evolution of Nitric Oxide Function: From Reactivity in the Prebiotic Earth to Examples of Biological Roles and Therapeutic Applications.Antioxidants (Basel). 2022 Jun 22;11(7):1222. doi: 10.3390/antiox11071222. Antioxidants (Basel). 2022. PMID: 35883712 Free PMC article. Review.
-
CO impedes superfast O2 binding in ba3 cytochrome oxidase from Thermus thermophilus.Proc Natl Acad Sci U S A. 2010 Dec 7;107(49):21010-5. doi: 10.1073/pnas.1008603107. Epub 2010 Nov 19. Proc Natl Acad Sci U S A. 2010. PMID: 21097703 Free PMC article.
-
Microbial ecology of the dark ocean above, at, and below the seafloor.Microbiol Mol Biol Rev. 2011 Jun;75(2):361-422. doi: 10.1128/MMBR.00039-10. Microbiol Mol Biol Rev. 2011. PMID: 21646433 Free PMC article. Review.
-
The evolution of respiratory O2/NO reductases: an out-of-the-phylogenetic-box perspective.J R Soc Interface. 2014 Sep 6;11(98):20140196. doi: 10.1098/rsif.2014.0196. J R Soc Interface. 2014. PMID: 24968694 Free PMC article. Review.
References
-
- Van der Oost J, de Boer A P N, de Gier J L, Zumft W G, Stouthamer A H, Spanning R J M. FEMS Microbiol Lett. 1994;121:1–10. - PubMed
-
- Saraste M, Castresana J. FEBS Lett. 1994;341:1–4. - PubMed
-
- Hendriks J, Gohlke U, Saraste M. J Bioenerg Biomembr. 1998;30:15–24. - PubMed
-
- Iwata S, Ostermeier C, Ludwig B, Michel H. Nature (London) 1995;376:660–669. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

