The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization
- PMID: 10611296
- PMCID: PMC24731
- DOI: 10.1073/pnas.96.26.14819
The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization
Abstract
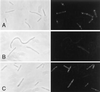
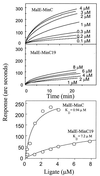
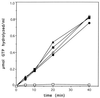
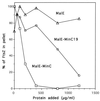
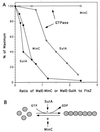
Positioning of the Z ring at the midcell site in Escherichia coli is assured by the min system, which masks polar sites through topological regulation of MinC, an inhibitor of division. To study how MinC inhibits division, we have generated a MalE-MinC fusion that retains full biological activity. We find that MalE-MinC interacts with FtsZ and prevents polymerization without inhibiting FtsZ's GTPase activity. MalE-MinC19 has reduced ability to inhibit division, reduced affinity for FtsZ, and reduced ability to inhibit FtsZ polymerization. These results, along with MinC localization, suggest that MinC rapidly oscillates between the poles of the cell to destabilize FtsZ filaments that have formed before they mature into polar Z rings.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

