Mice cloned from embryonic stem cells
- PMID: 10611324
- PMCID: PMC24759
- DOI: 10.1073/pnas.96.26.14984
Mice cloned from embryonic stem cells
Abstract
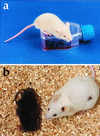
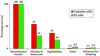
Cloning allows the asexual reproduction of selected individuals such that the offspring have an essentially identical nuclear genome. Cloning by nuclear transfer thus far has been reported only with freshly isolated cells and cells from primary cultures. We previously reported a method of cloning mice from adult somatic cells after nuclear transfer by microinjection. Here, we apply this method to clone mice from widely available, established embryonic stem (ES) cell lines at late passage. With the ES cell line R1, 29% of reconstructed oocytes developed in vitro to the morula/blastocyst stage, and 8% of these embryos developed to live-born pups when transferred to surrogate mothers. We thus cloned 26 mice from R1 cells. Nuclei from the ES cell line E14 also were shown to direct development to term. We present evidence that the nuclei of ES cells at G(1)- or G(2)/M-phases are efficiently able to support full development. Our findings demonstrate that late-passage ES cells can be used to produce viable cloned mice and provide a link between the technologies of ES cells and animal cloning. It thus may be possible to clone from a single cell a large number of individuals over an extended period.
Figures



References
-
- Willadsen S M. Nature (London) 1986;320:63–65. - PubMed
-
- Campbell K H S, McWhir J, Ritchie W A, Wilmut I. Nature (London) 1996;380:64–66. - PubMed
-
- Schnieke A E, Kind A J, Ritchie W A, Mycock K, Scott A R, Ritchie M, Wilmut I, Colman A, Campbell K H. Science. 1997;278:2130–2133. - PubMed
-
- Wilmut I, Schnieke A E, McWhir J, Kind A J, Campbell K H S. Nature (London) 1997;385:810–813. - PubMed
-
- Cibelli J B, Stice S L, Golueke P, Kane J J, Jerry J, Blackwell C, Ponce de Léon F A, Robl J M. Science. 1998;280:1256–1258. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

