Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities
- PMID: 10611369
- PMCID: PMC24804
- DOI: 10.1073/pnas.96.26.15239
Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities
Abstract
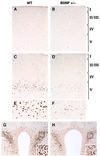
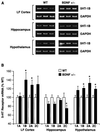
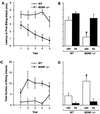
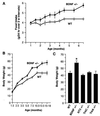
Brain-derived neurotrophic factor (BDNF) has trophic effects on serotonergic (5-HT) neurons in the central nervous system. However, the role of endogenous BDNF in the development and function of these neurons has not been established in vivo because of the early postnatal lethality of BDNF null mice. In the present study, we use heterozygous BDNF(+/-) mice that have a normal life span and show that these animals develop enhanced intermale aggressiveness and hyperphagia accompanied by significant weight gain in early adulthood; these behavioral abnormalities are known to correlate with 5-HT dysfunction. Forebrain 5-HT levels and fiber density in BDNF(+/-) mice are normal at an early age but undergo premature age-associated decrements. However, young adult BDNF(+/-) mice show a blunted c-fos induction by the specific serotonin releaser-uptake inhibitor dexfenfluramine and alterations in the expression of several 5-HT receptors in the cortex, hippocampus, and hypothalamus. The heightened aggressiveness can be ameliorated by the selective serotonin reuptake inhibitor fluoxetine. Our results indicate that endogenous BDNF is critical for the normal development and function of central 5-HT neurons and for the elaboration of behaviors that depend on these nerve cells. Therefore, BDNF(+/-) mice may provide a useful model to study human psychiatric disorders attributed to dysfunction of serotonergic neurons.
Figures

References
-
- Eaton M J, Staley J K, Globus M Y, Whittemore S R. Dev Biol. 1995;170:169–182. - PubMed
-
- Siuciak J A, Boylan C, Fritsche M, Altar C A, Lindsay R M. Brain Res. 1996;710:11–20. - PubMed
-
- Baumgarten H G, Grozdanovic Z. Pharmacopsychiatry. 1995;28, Suppl. 2:73–79. - PubMed
-
- Hen R. Neuron. 1996;16:17–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

